Translate this page into:
Validation of immunohistochemical tests performed on cytology cell block material: Practical application of the College of American Pathologists’ guidelines
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The advent of fiberoptic endoscopy with biopsy has revolutionized procurement of specimens from deep sites. This has translated into more cytologic specimens whereby the material is limited and best handled by cytology laboratory staff. While the diagnosis of the pathologic process is of utmost importance, there is increasing expectation that the diagnosis be specific and accurate as not to require additional biopsy for initiation of treatment. This expectation has driven demand in immunohistochemical (IHC) and molecular studies conducted specifically on material processed as cytology specimens. The Clinical Laboratory Improvement Amendments of 1988 requires laboratories in the United States of America to verify the performance of patient tests. Due to varying laboratory practices with respect to validation of IHC assays, the College of American Pathologists introduced guidelines for analytic validation of IHC tests. These guidelines address how to perform validation by recommending the number of cases in the validation set, comparator concordance, and when to revalidate. The main thrust of the guidelines is based on formalin-fixed paraffin-embedded tissue with only one expert consensus opinion referring to validation of IHC tests on cytology specimens which delegates to the medical director, the determination of number of positive and negative cases to be tested. This article will outline how an academic center approaches validation of IHC studies performed on cytology cell block specimens using the College of American Pathologists guidelines. A stepwise approach from selection of antibodies to validate followed by building the validation panel and evaluating the stain results for concordance against the gold standard of histology tissue specimen will be described. A rationale for dealing with discordant results and future innovations will conclude the report.
Keywords
Cell block
clinical laboratory improvement amendments
College of American Pathologists
cytology
immunohistochemistry
laboratory practice
validation
INTRODUCTION
Cytology cell blocks have assumed increased importance due to the increased demand for specific diagnosis beyond the general categories of benign, malignant, or inflammatory. There is, however, little appreciation of the different methods of creating cytology cell blocks which can lead to significant variation in the performance of immunohistochemical tests. The Clinical Laboratory Improvement Amendments (CLIA) of 1988 requires laboratories in the United States of America (USA) to verify the performance of patient tests. The College of American Pathologists (CAP) in a questionnaire-based survey of 727 laboratories in the USA, found that a significant proportion (63%) of respondents did not have written procedures for validation of immunohistochemical test of cytologic material.[1] Of those laboratories that claimed to validate the immunohistochemical tests, there was wide variation of the validation process. This led to the publication of CAP guidelines for analytic validation of immunohistochemical assays which also includes a brief reference to cytology specimens.[2]
The objective of a cell block is to collect all the cells in the suspension for subsequent processing through paraffin embedding and microtome cut sections stained with hematoxylin and eosin (H and E). The cell concentration is facilitated by centrifugation to form a pellet. The pellet may be collected in a collodion bag which is then tied off before formalin fixation and paraffin embedding (FFPE). Molten agar can be used to bind the pellet of cells before solidifying the mixture by placing it in the refrigerator. This is then processed by FFPE before H and E sections examined. The plasma–thrombin method is the most widely utilized method of generating a cytology cell block. This is due to the relative ease of stirring one drop of plasma to the centrifuged cell pellet before addition of one drop of thrombin (BD Thermo Scientific Cytoblock). The soft clot is then transferred to a cassette for FFPE and sectioning. The Cellient Automated Cell Block System (Hologic) diverges from centrifugation to using vacuum-assisted filtration to deposit the cells directly into the cassette. Table 1 summarizes the pros and cons of these methods.[345678]
| Cell block method | Advantages | Disadvantages | Use | ICC | Molecular studies |
|---|---|---|---|---|---|
| Plasma thrombin (BD)[34] | Simple, low cost Easy availability of reagents Optimal cytomorphology Clean background for ancillary studies | Cross contamination from plasma and thrombin possible Uneven concentration of cells | Suitable for serous fluids, washings, urines, FNAs, LBC, and any cell suspension not fixed in formalin | Optimal results | Optimal results |
| Automated (hologic)[7] | Good cellular yield* Uniformly distributed cells at face of block Improved cellular architecture and nuclear features Consistent results Reduced procedural time No cross contamination Minimal cell loss | Alcohol medium potentially compromises the comparison of IHC results which are generally based on FFPE Expensive machines and consumables Requires trained staff for cutting thin blocks | Limited studies* Useful in low-cellularity specimens Useful in cervical LBC | Limited data Possible false negatives for hormone receptors due to alcohol fixation | High quality of DNA and RNA |
| Agar method[356] | Inexpensive Better orientation of cell block material using marker | Meticulous attention to step for even melting of the gel which is prone to exploding/ while reheating/microwaving Heat-related artifacts possible if not cooled as recommended (exaggerated vacuoles, dense cytoplasm, shrunken cells, frayed cytoplasm) | For any fluid or FNA | Optimum results for cytoplasmic and nuclear antigens | Suitable |
| Shidham’s method[8] | Inexpensive Better orientation of hypocellular samples while making cell block; use of AV marker allows location of block facing surface | Requires trained staff for preparing and cutting blocks | Standardized for cervical LBC but can be used for any fluid or FNA | Optimal results | Optimal results |
FNAs: Fine-needle aspiration, LBC: Liquid-based cytology, IHC: Immunohistochemical, FFPE: Formalin fixation and paraffin embedding, AV: Anjani-Vinod. *Depends on cellularity of sample
It is evident that there are multiple variables in creating a cytology cell block. This includes the time the cytology material spends in the fixative solution, the type of fixative used (ethanol, methanol, isopropanol, formalin, and mixtures of these), protein contaminants in the form of plasma and thrombin, and heat and cooling artifacts on cells in agar cell blocks. The cell block is therefore, potentially, a unique product of the laboratory in which it was created. This is a significant factor pertinent to the performance of validation studies.
COLLEGE OF AMERICAN PATHOLOGISTS GUIDELINES ON ANALYTIC VALIDATION OF IMMUNOHISTOCHEMICAL STUDIES
The CLIA charges laboratories reporting patient results to verify their assays in a stepwise manner through assay optimization, validation, and ongoing monitoring for consistency. The CAP questionnaire-based laboratory survey[1] found “significant interlaboratory variation in validation practices and revealed that many laboratories do not follow consistent procedures when validating immunohistochemical assays.” The CAP, therefore, published guidelines with the specific objective of helping pathology laboratories comply with validation and revalidation requirements. Five recommendations and nine expert consensus opinions were issued to guide the immunohistochemical test validation procedures. These guidelines pertain mainly to surgical material except for one expert consensus opinion which addresses cytology specimens by stating that “… laboratories should test a sufficient number of such cases to ensure that assays consistently achieve expected results. The laboratory medical director is responsible for determining the number of positive and negative cases and the number of predictive and nonpredictive markers to test.”
PROCEDURE FOR APPLICATION OF COLLEGE OF AMERICAN PATHOLOGISTS IMMUNOHISTOCHEMICAL STUDIES VALIDATION GUIDELINES TO CYTOLOGY CELL BLOCKS
Our first priority was to select a subset of antibodies to validate on cytology cell blocks as validation of all antibodies used was impractical due to limited material, time, and expense of the validation process. Table 2 shows a breakdown of specimen types that compose our cytology sample. The lung panel (TTF-1, p40, p63, AE1/3, CK7, CD56, synaptophysin, and chromogranin) was the most frequently utilized diagnostic immunohistochemical study in our laboratory with a frequency of 1016 tests for TTF-1 and 143 tests for chromogranin. In addition, Ki-67 (219 cases) and CK8/18 (188 cases) were also within the frequency of most utilized immunohistochemical stains and hence were included into our antibody selection.
| Organ/site | Number over 8-month period |
|---|---|
| Body fluids | 949 |
| Lymph nodes | 820 |
| Lung* | 421 |
| Thyroid | 394 |
| Pancreas | 101 |
| Liver | 49 |
*Lung was the most frequent organ for which immunohistochemical studies were performed
Ten consecutive lung resection specimens were used for validation studies and sampling occurred within 45 minutes of removal from the patient. The lesion in the fresh resection specimen was scraped twice. The first scraping was suspended in alcohol-based fixative (CytoRich Red) and the second scraping was suspended in 10% formalin solution, both were processed as cell block material using the plasma–thrombin method. In addition, a section of the lesion was also procured for processing as FFPE tissue. All materials were handled as per existing standard operating procedures. The cytology cell block material served as both positive and negative control; the negative control being the resident benign population of cells such as macrophages, bronchial cells, lymphoid cells, as well as nonreactive malignant cells.
Immunohistochemical tests on cytology cell block material were performed in the same manner as that validated for the surgical material which had >90% concordance with the morphologic gold standard. The results of the validation study scored percentage of cells staining in the surgical and cytology material as follows (0%, 1%–25%, 26%–50%, 51%–75%, and >75%) except for Ki67 which was scored at decile intervals (<0%, 1%–10%, 11%–20%, etc). Concordance was achieved if the difference in staining was within the same quartile or in case of Ki-67, within the same decile interval. In addition, intensity of staining compared to control FFPE tissue was scored on cytology material using a binary method of equal or greater than control (score of 1) or less than control (score <1).
RESULTS
Table 2 presents the breakdown of cytology specimens received in our department over the 8-month period preceding our validation study. Although exfoliative specimens from body fluids and lymph node aspirates were more numerous, the majority of these specimens did not need immunohistochemical studies (IHC) for characterization of the pathologic process. Specimens from the lungs were the ones that most frequently utilized immunohistochemical tests for diagnosis.
The performance characteristics of the validation tissue panel obtained from ten resected lung specimens are illustrated in Table 3. CK8/18, CD56, chromogranin, synaptophysin, p40, and TTF-1 showed equal intensity and proportional staining of neoplastic cells regardless of whether the cell blocks were fixed in alcohol (CBA) or formalin (CBF). Figure 1 illustrates the TTF-1 staining in the three types of preparations.
| Antibody (source) | Quartile difference from FFPE (# of cases) | Intensity difference from FFPE (# of cases) |
|---|---|---|
| AE1/3 (Leica, multikeratin mouse ascitic fluid) | 0 (6); 1 (−CBF, −CBA) | 0 (6), <1 (−CBF) |
| CK7 (Leica RN7, mouse monoclonal) | 0 (7); 1(−CBF) | 0 (8) |
| CK8/18 (Leica, 5D3, mouse monoclonal) | 0 (7) | 0 (7) |
| CD56 (Leica, CD564 mouse monoclonal) | 0 (3) | 0 (3) |
| Chromogranin | 0 (3) | 0 (3) |
| Synaptophysin (Leica, 27G12 mouse monoclonal) | 0 (3) | 0 (3) |
| p40 (Biocare, Rabbit polyclonal) | 0 (9) | 0 (9) |
| p63 (Biocare, BC4A4 mouse monoclonal) | 0 (8); 1 (−CBF, −CBA) | 0 (7); 1 (−CBA) |
| TTF-1 (Leica, SPT24 mouse monoclonal) | 0 (10) | 0 (10) |
FFPE: Formalin-Fixed Paraffin-Embedded histology tissue which serves as the control, CBF: Formalin-fixed cell suspension processed by plasma-thrombin method into a cell block, CBA: Alcohol-fixed cell suspension (CytoRich) processed by plasma thrombin into a cell block, − Denotes decrease in proportion of cells stained within the CBF/CBA preparations
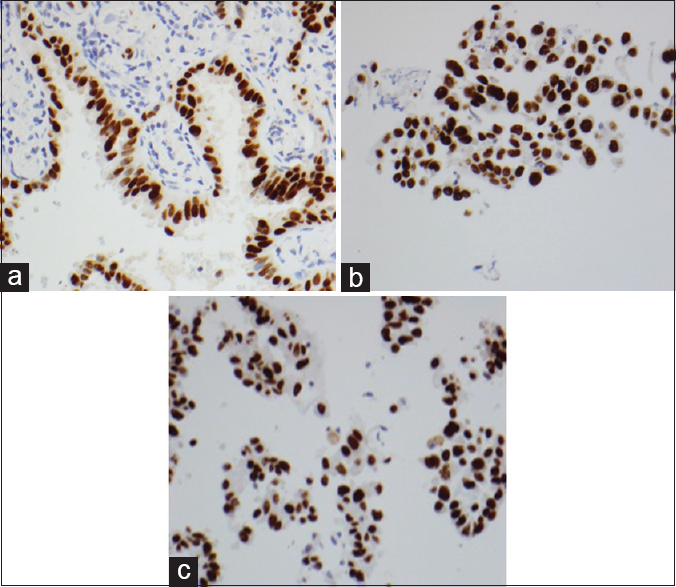
- Equal intensity and proportional staining of TTF-1 in tumor cells, formalin-fixed paraffin-embedded, ×400 (a), cell blocks fixed in formalin, ×400 (b), and cell blocks fixed in alcohol, ×400 (c)
AE1/3, p63, and CK7 revealed variation in proportion and intensity of staining of neoplastic cells. Compared with the control FFPE tissue, the proportion of AE1/3-stained tumor cells in one of seven specimens fell into a lower quartile in both CBA and CBF. Compared to the FFPE gold standard, less intense AE1/3 staining of tumor cells was identified in one CBF with no difference in AE1/3 staining intensity identified in any of the CBA [Table 3].
For p63, one out of nine cases showed 1%–25% staining of tumor cells in FFPE tissue compared to no staining of tumor cells in both CBA and CBF. The intensity of the other seven cases was comparable across the preparations with only one CBA specimen showing less intense staining than FFPE tissue [Table 3].
For CK7, in one out of eight specimens, tumor cell staining in the FFPE fell into a lower quartile than tumor cells in the CBF. There was a comparable proportion of staining in all CBA. No variation in intensity of staining was identified across the specimen preparation types [Table 3].
The Ki-67 results were particularly subject to variation in both proportion and intensity of staining [Table 4]. Compared with the control FFPE tissue, the proportion of tumor cell staining was lower in the CBA and CBF of all eight cases. The proportion variation of staining of the cells ranged from 10% to 20%. The intensity of staining of the cell block material was lower (six out of eight) compared to FFPE material. This appeared independent of whether the cell block was from alcohol or formalin-fixed material. Figure 2 illustrates the Ki-67 staining in the three types of preparations.
| Validation case number (diagnosis) | CBF decile difference from FFPE | CBF intensity difference from FFPE | CBA decile difference compared with FFPE | CBA intensity difference from FFPE |
|---|---|---|---|---|
| V1 (neuroendocrine) | −1 (20-30) | 1 | −2 (20-30) | <1 |
| V2 (adenocarcinoma) | −1 (0-10) | N/A | −1 (0-10) | N/A |
| V3 (metastatic carcinoma) | 0 (10-20) | 1 | −1 (10-20) | <1 |
| V5 (adenocarcinoma) | −2 (20-30) | <1 | −1 (20-30) | 1 |
| V6 (squamous cell carcinoma) | −1 (10-20) | <1 | 0 (10-20) | 1 |
| V7 (adenocarcinoma) | 0 (40-50) | 1 | −2 (40-50) | 1 |
| V11 (adenocarcinoma) | 0 (0-10) | 1 | −1 (0-10) | 1 |
| V12 (adenocarcinoma) | <1 (0-10) | <1 | <1 (0-10) | <1 |
FFPE: Formalin-fixed paraffin-embedded histology tissue which serves as the control, Ki- 67 was scored as tenths with <0%, 1%-10%, 11%-20%, etc. The proportion of cells stained is either equal to (hence difference is 0) or less than those stained in the FFPE tissue. The latter is denoted by the (−) in front of the decile difference such that -2 amounts to 2 decile decreased staining compared with FFPE tissue. The intensity was graded as 1 if it was equal to or greater than the control FFPE tissue, <1% if it was less intense than control FFPE staining and 0 if there was no staining. CBA: Cell Blocks fixed in alcohol
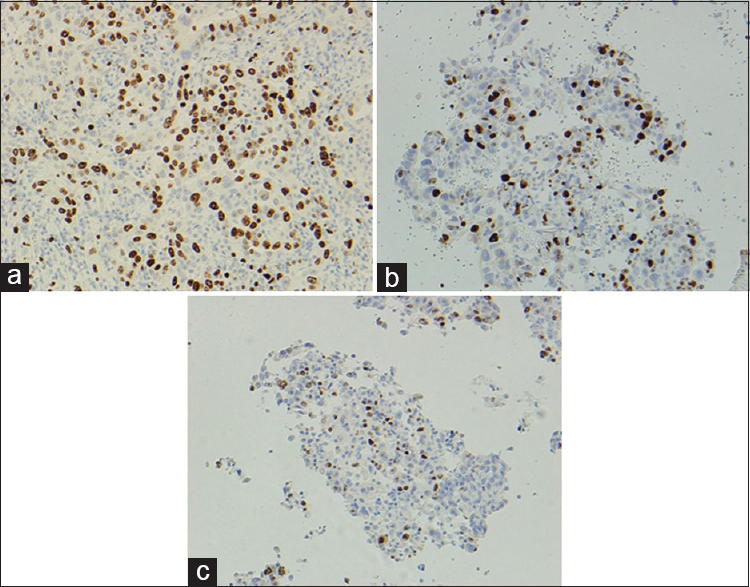
- Difference in intensity and proportion of staining of Ki-67 in tumor cells, formalin-fixed paraffin-embedded, ×200 (a), cell blocks fixed in formalin, ×200 (b) and cell blocks fixed in alcohol, ×200 (c)
DISCUSSION
With the exception of Ki-67, IHC on cytology cell blocks created using plasma–thrombin method performed comparatively well with reference to the results of previously established immunohistochemical protocols for surgical material. There was broad concordance within the same quartile interval of the proportion of cells staining and intensity of staining across the alcohol-fixed cell block, formalin-fixed cell block, and FFPE material. Intensity of staining (both nuclear and cytoplasmic/membranous) was broadly equivalent across the preparation types. Although there was occasional variation in intensity and proportion of neoplastic cells in cell block compared to FFPE material with reference to AE1/3, CK7, and p63 antibodies these did not preclude interpretation and could be explained by tumor cell heterogeneity rather than processing methods.
The performance of Ki-67 was particularly variable in the cell block material. The degree of variation was interpreted to be too wide for meaningful clinical use. Tumor heterogeneity as well as plasma–thrombin cell block preparation could contribute to the differences observed. We, therefore, cautiously interprete Ki-67 on cell block materials with the realization that it underestimates the proliferative index and that no staining might be due to loss of reactivity of the antibody.
There are various reports whereby the results of nuclear staining are dependent on the clone of antibody used[9] and the fixative. Apart from the Ki-67 antibody clone, all the other antibody clones used in our validation study performed equally well with respect to nuclear positivity of the stain in alcohol-based CytoRich Red and 10% formalin fixatives.
In theory, where there is <90% concordance, the McNemar test may be used to determine whether there is a significant positive or negative bias between the test and reference material. This would then inform mitigation processes such as antigen retrieval procedure and variation in incubation times. The McNemar test requires a large number of samples to run the two by two table and is therefore impractical in our case where only ten specimens comprised the validation set. Changes to the immunohistochemical protocol seem feasible, but the added effort of optimization and revalidation as well as programming of the immunohistochemistry instrument for a varying protocol specifically used for cytology cell block make it a less attractive option.
As noted previously, we did not validate all the antibodies used in histology specimens because of lack of specific tissue type, expense, and time of performing antibody validation tests. Our cost analysis revealed price per slide ranging from $8.34 (majority of antibodies) to $15.20 (p63 antibody) with technical time estimated at $14 per slide based on 30 minutes of time utilized. The total cost amounted to $4000 for the ten cases that were validated. The cost burden would increase significantly if prognostic markers (such as HER2/neu, ER, and PR) are to be validated. This is because the study would require the more stringent criteria of including twice the number of specimens used for diagnostic antibody markers.[2]
FUTURE INNOVATIONS
Efforts toward standardization would very likely lower the cost and increase the range of antibodies that could be validated. Suspension of cells from all needle rinses in formalin would eliminate the duplication of validation procedures for alcohol-fixed cells. Automation of cell block creation would similarly minimize preanalytic variables influencing cell block performance for cytology. The Cellient Automated Cell Block System is an example of such a system; however, it has the distinct disadvantage of processing only one cell block at a time. Validation sets such as cytology cell block microarrays and cell culture pellets would allow laboratories to share validation sets and enable antibody optimization studies before the validation process. Progress toward standardization of cytology cell blocks will have added value of enhancing the validity of other ancillary tests such as in situ hybridization and molecular tests.
SUMMARY AND CONCLUSION
There is increased expectation that a cytology specimen diagnosis would be precise and definitive to allow treatment and prognostication. IHC are central to diagnosis, and it behooves laboratories to validate their tests so as to ensure accuracy.
Our stepwise validation method is based on an academic university cytopathology laboratory practice [Figure 3]. The first step is identification of the most frequent cytology specimens on which diagnostic IHC are performed. This informs on the type of specimen(s) to use to generate tissue for a validation panel. Using standard laboratory procedures, we were able to validate antibodies tested which were in use in the histology laboratory and which demonstrated >90% concordance with morphologic gold standard. Non validated immunohistochemical stains are still used on cell blocks, but these are interpreted cautiously as advised by the CAP guideline. The guidelines also state that a disclaimer should be used in the report to draw attention to this.
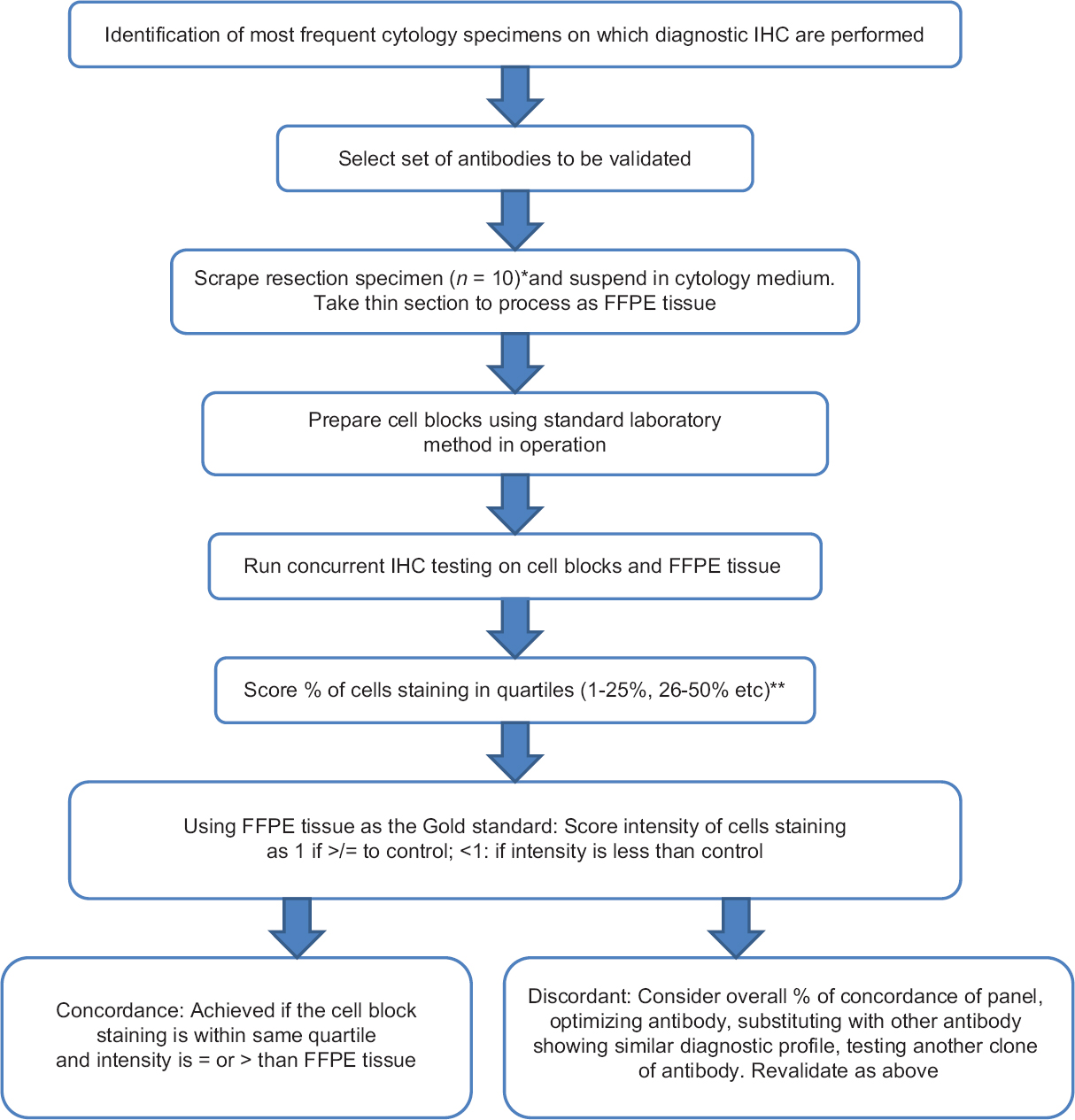
- Flow diagram of stepwise validation of immunohistochemical studies in cytology cell blocks. *Number of specimens in panel at discretion of Medical Director. **Ki-67 was scored in decile (see main text)
Innovations of standardizing preanalytical elements for cell blocks would reduce the cost of validation studies, increase the range of validated antibodies, allow for interlaboratory utilization of standard material, and add value for validation of other ancillary testing that is done on cell blocks such as molecular and in situ hybridization studies.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Dr. Swati Satturwar, Dr. Renuka Malenie, Dr. Ann Sutton, Dr. Diana Dai and Dr. F. Zahra Aly have contributed in collection of cases, analysis of data and writing of manuscript. Dr. Ann Sutton and Dr. F. Zahra Aly edited the manuscript. All authors were involved in conceptualizing the study.
ETHICS STATEMENT BY ALL AUTHORS
Not applicable.
LIST OF ABBREVIATIONS (In alphabetic order)
CAP – College of American Pathologists
CBA – Cell blocks fixed in alcohol
CBF – Cell blocks fixed in formalin
CLIA – Clinical Laboratory Improvement Amendments
FFPE – Formalin-fixed paraffin-embedded
H and E – Hematoxylin and eosin
ICC – Immunocytochemistry
IHC – Immunohistochemical studies
USA – United States of America.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Immunohistochemistry validation procedures and practices: A College of American Pathologists survey of 727 laboratories. Arch Pathol Lab Med. 2013;137:19-25.
- [Google Scholar]
- Principles of analytic validation of immunohistochemical assays: Guideline from the college of american pathologists pathology and laboratory quality center. Arch Pathol Lab Med. 2014;138:1432-43.
- [Google Scholar]
- Cell blocks in cytopathology: A review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. 2014;25:356-71.
- [Google Scholar]
- Utility of the thromboplastin-plasma cell-block technique for fine-needle aspiration and serous effusions. Diagn Cytopathol. 2009;37:86-90.
- [Google Scholar]
- Diagnostic value of processing cytologic aspirates of renal tumors in agar cell (tissue) blocks. Acta Cytol. 2010;54:587-94.
- [Google Scholar]
- AgarCyto: A novel cell-processing method for multiple molecular diagnostic analyses of the uterine cervix. J Histochem Cytochem. 2000;48:709-18.
- [Google Scholar]
- Automated cellient(™) cytoblocks: Better, stronger, faster? Cytopathology. 2014;25:372-80.
- [Google Scholar]
- Cell block preparation from cytology specimen with predominance of individually scattered cells. JVis Exp 2009 pii: 1316
- [Google Scholar]
- Demonstration of CDX2 is highly antibody dependant. Appl Immunohistochem Mol Morphol. 2013;21:64-72.
- [Google Scholar]








