Translate this page into:
Abstracts for the 59th Annual Scientific Meeting (November 2011) by American Society of Cytopathology (ASC) at Baltimore, MD, USA
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
These are peer-reviewed poster-platform submissions finalized by the Scientific Program Committee. A total of 153 abstracts (14 Platforms [PP1 through PP14] & 139 Posters [1 through 139]) were selected from 161 submissions to be considered for presentation during November 4 – 8, 2011, at the Hilton Baltimore Hotel, to pathologists, cytopathologists, cytotechnologists, residents, fellows, students, and other members of cytopathology-related medical and scientific fields.
Keywords
Abstracts
American society of cytopathology
ASC
cytopathology
cytology
87: Use of the Cellient™ automated cell block system to process small surgical biopsy specimens that may not survive traditional processing
Dawn Underwood, MS, CT(ASCP), Alisha Cotman, Joseph Begany, PA(ASCP), Brad Skilton, PA(ASCP), Linda McDonald, HT(ASCP), EM(EMSA), Jennifer Brainard, MD
Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio
Introduction: Small biopsy specimens with minimal visible tissue are a challenge to process. At our institution, these samples are particularly common in gynecological pathology. Samples ≤ 1 mm in one dimension or those that consist predominantly of mucus are vulnerable to loss during processing. In our experience, approximately one-third of these cases are insufficient for diagnosis. The Cellient™ automated cell block system is a novel method for creating cell blocks from cytology samples, using controlled vacuum to deposit material onto a filter, followed by infiltration of the sample with reagents and paraffin. Cellular material is concentrated to maximize cell yield in an automated and more consistent manner when compared to traditional cell block methods. We decided to see if the use of the Cellient™ System to process small gynecological biopsies would facilitate diagnosis and result in fewer unsatisfactory samples.
Materials and Methods: Small biopsies that may not survive processing are defined as samples that measure ≤ 1 mm in one dimension or those that consist predominantly of mucus, with scant visible tissue. Traditional processing involves filtering formalin-fixed samples into a nylon mesh biopsy bag, followed by a transfer of the contents of the bag to a histowrap paper. The histowrap paper is placed into a cassette for further processing and paraffin embedding. For Cellient™ cell block processing, the formalin-fixed biopsy samples were washed twice with 20 ml of CytoLyt and placed in a ThinPrep® vial containing PreservCyt solution. The samples, without visible tissue fragments, were directly transferred from the vial into the cassette / filter assembly by the instrument. If tissue fragments were visible, they were manually loaded by the cytopreparatory personnel into the cassette / filter assembly. The sample was augmented by aspiration of additional cellular material from the ThinPrep® vial. The instrument processed the sample and infused paraffin to produce a cell block. The blocks were cut and stained with H and E. At least two tissue sections were present on each of the two levels. All slides in the study were reviewed by one gynecological surgical pathologist.
Results: In 2010, the data for 422 gynecological surgical biopsies with minimal tissue were processed, using the traditional methods that were available. A diagnosis was rendered in 279 (66%) cases. The remaining 143 (34%) were insufficient for evaluation. Cellient™ cell block processing was introduced during a one-month study period, in April 2011. During this time, 135 samples were processed, including 111 endocervical curettings (ECC), 19 endometrial biopsies (EMB), two endometrial curettings, and three cervical biopsies (CXBX). Twenty-six cases (19%) processed using the Cellient™ System were insufficient for evaluation (18 ECC, 6 EMB, and 2 CXBX). Nineteen cases were manually processed and all were diagnostic. Dysplastic squamous cells were identified in seven ECC (three CIN 2, two CIN 1, and two squamous dysplasias that could not be further classified). All endometrial and cervical biopsy diagnoses were benign.
Conclusions: Rates of unsatisfactory gynecological biopsies decreased from 34% with traditional processing to 19% using the Cellient™ System, during the study period. The Cellient™ method concentrates the cellular material in the center of the microscopic slide, facilitating microscopic evaluation. Close communication between surgical pathology, histology, and cytology is necessary for optimal specimen handling.
88: Constructing a twenty-first century cytology laboratory
Janie Roberson, SCT(ASCP), Allison Wrenn, CT(ASCP), John Poole, AIA, Andrew Jaeger, Isam Eltoum, MD, MBA
Pathology, University of Alabama at Birmingham, Birmingham, Alabama
Introduction: Constructing or renovating a laboratory can be both challenging and rewarding. UAB Cytology (UAB CY), recently undertook a project to relocate from a building constructed in 1928 to a new space. UAB CY is part of an academic center serving two Cytotechnology programs, two Fellowships, and support a staff active in research and publication. Our objectives were to provide a safe, aesthetically pleasing space, and gain efficiencies through Lean processes.
Materials and Methods: The phases of any laboratory design project are planning, schematic design (SD), design development (DD), construction documents (CD) and Construction. Laboratory personnel are most critical in the planning phase. During this time stakeholders, relationships, budget, square footage, and equipment are identified. Equipment lists, including what will be relocated, newly purchased, and projected for future growth ensure that utilities are matched to the need. A chemical inventory is essential for providing adequate storage space. Regulatory and safety requirements are discussed determining that the wet laboratory space be designated as Bio safety level 2 (BSL2). Tours and high level process flow diagrams help architects and engineers understand the daily laboratory work. Future needs are addressed through a questionnaire, which identifies the potential areas of growth and technological change. Throughout the project, decisions are driven by the data from the planning phase. During the SD phase, objective information from the first phase is used by architects and planners to create a general floor plan. This is the basis of a series of meetings to brainstorm and suggest modifications. DD brings more detail to the plans with engineering, casework, equipment specifics, and finishes. Design changes must be completed at this phase. The next phase, CD, takes the project from the laboratory purview into a purely technical mode. Construction documents are used by the contractor for the bidding process and ultimately the construction phase.
Results: The project fitted out a total of 9,000 square feet; 4,000 laboratories and 5,000 office / support. The laboratory space included areas for prep, CT screening, sign out, and imaging. The adjacent space housed faculty offices and conferencing facilities. Transportation time was reduced (waste removal) by a Pneumatic Tube System, specimen drop window to the prep laboratory, and a pass through window to the screening area. The open screening and prep areas allowed visual management control. Efficiencies were gained by ergonomically placing the CT Manual and Imaging microscopes and computers in close proximity, also facilitating a paperless workflow for additional savings. Logistically, closer proximity to Surgical Pathology maximized the natural synergies between the areas.
Conclusions: Laboratory construction should be a systematic process based on sound principles for safety, high quality testing, and finance. Our detailed planning and design process could be a model for others undertaking similar projects.
89: Trends in practice before and after implementation of EUS-FNA: A disruptive innovation effect
Evans Alston, MD, Janie Roberson, CT(ASCP), Isam Eltoum, MD, MBA
Pathology, University of Alabama at Birmingham, Birmingham, Alabama
Introduction: The CAP Futurescape Forum has introduced the concept of disruptive innovation as a means to assess the impact of technology on pathology practice. Little has been reported on changes in the volume and types of pathology specimens, spectrum of pancreatic disease, and the accuracy of cytologic diagnosis, before and after implementation of EUS-FNA. In this study, we assessed these parameters before and after implementation of EUS-FNA, in a large academic center. We also attempted to determine if the impact of EUS-FNA cytology on replacing tissue biopsy in the diagnosis of pancreatic disease fulfils the Christensen's criteria of disruptive innovation.
Materials and Methods: Electronic records of 20 years of pancreatic specimens, both cytological and histological evaluations (10 years before and 10 years after implementation of EUS-FNA) were reviewed. For these two periods, the following parameters were compared: The total and annual average of each and every type of pancreatic specimen, diagnostic categories, and accuracy of cytological diagnoses, using histology as the gold standard. The chi-square test was used as appropriate with the difference considered significant at P < 0.05. Disruptiveness of the cytology relevant to biopsy was assessed by comparing the utilization trend and the accuracy of diagnosis over time.
Results: Over 20 years, there was a gradual increase of all pancreatic specimens [Figure 1]: The mean annual volume (SD) of cytological specimens was 24 (11) before versus 231(10) after implementation (9 non-EUS-FNA, 222 EUS-FNA), and that of histological specimens was 97 (42) before versus 377 (148) after implementation. The average annual percent of cases managed following cytology alone was 19% (10) before versus 51% (8) after implementation. In contrast, those managed after histology alone was 56% before versus 23% after implementation. Non-EUS FNA decreased from 36% to 1% of the total cytological pancreatic specimens. Out of all the pancreatic surgical pathology specimens, the total number of needle biopsies decreased from 7 to 1%; and the other biopsy types from 29 to 9%. Whipple procedures increased from 19 to 49%, while partial resections remained the same: 44 versus 41%. More definitive diagnoses such as categorization of neoplastic and cystic diseases were observed after implementation. A significant reduction in unsatisfactory (7 vs. 1%), atypical (16 vs. 4%), and suspicious (16 vs. 3%) cytological diagnostic category rates was noted. The accuracy of the cytological diagnosis significantly improved; for ductal carcinoma, the sensitivity (C.I.) and specificity (C.I.) were 55% (38 – 70%) and 78% (58 – 89%) before versus 88% (84 – 91%) and 96% (93 – 98%) after, respectively. The diagnostic odds ratio (C.I) for a positive cytology was 4.0 (1 – 13) compared to 200 (94 – 422) before and after implementation, respectively.
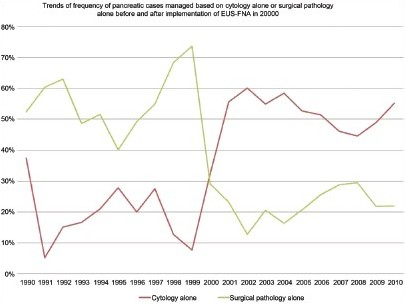
Conclusions: EUS-FNA has greatly improved the accuracy of cytological diagnosis, reduced the number of indeterminate diagnoses, and largely replaced the need for tissue biopsy. Given the cost and simplicity as compared to tissue biopsy, this trend represents a disruptive innovation effect.
90: Does cell block quality need to be sacrificed for turn-around time? The impact of rapid microwave processing on cytomorphological and immunohistochemical characteristics
Rebecca Heintzelman, MD, Hillary Kimbrell, MD, Fernando Garcia, MD, Xiaoli Chen, MD
Department of Pathology, Drexel University College of Medicine, Philadelphia, Pennsylvania
Introduction: Processing cell blocks with traditional tissue processors can delay the turn-around time (TAT) of cytology cases. Microwave tissue processors can decrease the processing time to less than an hour, resulting in better patient care, although there are no studies evaluating the appropriate time of fixation and the effect on cellular morphology. We have undertaken this study to evaluate whether processing cell blocks on a microwave processor adversely affects cytomorphology or immunoreactivity.
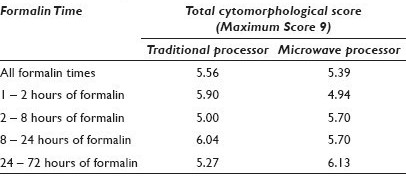
Materials and Methods: Sixty-four effusion fluid specimens were collected consecutively and divided evenly into two blocks, one processed on the traditional processor and the other using the microwave. Each block was assigned to a formalin fixation time of 1 – 2 hours, 2 – 8 hours, 8 – 24 hours, or 24 – 72 hours. Hematoxylin and Eosin (H and E) stained sections were prepared from each block and randomized. Each slide was blindly scored on a scale of 0 – 3, with regarf to the nuclear, cytoplasmic, and cell membrane details of the mesothelial cells by three reviewers. Each reviewer was also asked to predict the processing method that was used. MOC31, calretinin, and cytokeratin 7 (CK7) immunostains were performed on 39 paired cases, using the Ventana Benchmark system. Each slide randomly contained both microwave and traditional sections from the same fluid. The reviewers blindly compared the extent and intensity of the staining (1 – 3 scale) for each section. The results were averaged among the three reviewers and compared across the two processing methods and more specifically within the different formalin fixation time periods, using the Chi Square method.
Results: The average nuclear, cytoplasmic, cell membrane, and total cytomorphological scores demonstrated no significant differences between the processing methods and the fixation times in any of the categories (P > 0.6) [Table 1]. Reviewers correctly identified the processing method and the majority of the time, ranging from 60 to 73%. Notably, the mesothelial cells in the microwave blocks demonstrated a fine nuclear chromatin pattern more often than a hyperchromatic one (52% vs. 18%); the reverse held true for traditionally processed blocks (45% vs. 32%) [Figure 1]. Retrospectively, this was noted to be a helpful feature utilized by the reviewers when predicting the fixation method. There was also concordance among the reviewers with regard to the extent and intensity of immunohistochemical staining between the two processing methods. Immunostaining intensity for CK7 was consistently 3+, regardless of the processing method and formalin fixation time. MOC-31 and calretinin staining intensity results are demonstrated in [Figures 2 and 3].
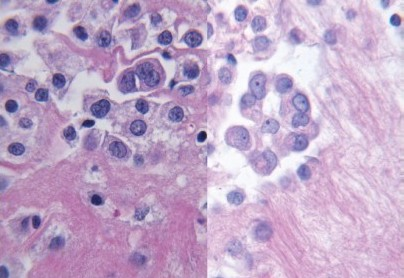
- Hyperchromatism from traditional processing (left) and fine nuclear chromatin from microwave processing (right) (H and E stain, 60×)
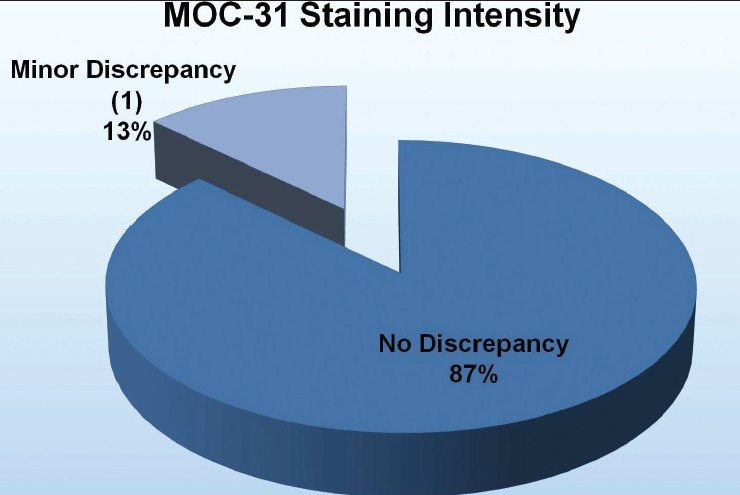
- Comparison of MOC-31 staining intensity between the microwave and traditional processing methods
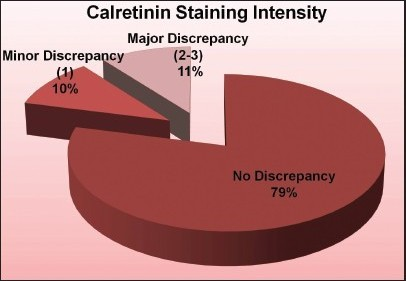
- Comparison of calretinin staining intensity between the microwave and traditional processing methods
Conclusions: 1) Overall, the total cytomorphological scores are similar, regardless of the processing method or formalin fixation time. 2) Microwave processing and formalin fixation time do not affect the immunohistochemical staining extent for CK7, MOC31, and calretinin. Staining intensity for CK7 and MOC-31 is unaffected and calretinin shows an 89% concordance rate between the two processing methods. 3) Microwave processed cell blocks tend to have mesothelial cells with finer chromatin instead of a hyperchromatic pattern, regardless of the fixation time. 4) Rapid-microwave processing can replace traditional processing methods as a viable option to make ‘same-day processing’ a reality for cytology and improve patient care.
91: Prospective use of glacial acetic acid in a grossly bloody Pap test: Reduction in hologic ThinPrep® rate of unsatisfactory specimens
Vickie Johncox, CT(ASCP), Vijayalakshmi Padmanabhan, MBBS, MD
Department of Pathology, Dartmouth Hitchcock Medical Center, Lebanon, New Hampshire
Introduction: Presence of blood, lubricant, mucous or inflammation can contribute to an unsatisfactory (unsat) Pap test (PT). When we recently changed vendors for Pap tests from BD SurePath® to Hologic ThinPrep®, one of our prime concerns was the unsat rate. The unsat rate for ThinPrep® has been reported at 1.2%; while with BD SurePath® our rate was 0.51%. Scant squamous cellularity in the presence of abundant fresh blood has been reported with ThinPrep® specimens. It has been postulated that excess blood in ThinPrep® samples may cause obstruction of membrane filters competing for the capture of epithelial cells producing a scantily cellular smear. Reprocessing with glacial acetic acid (GAA) for an unsat PT, a process that takes time, has been approved for improving adequacy. The aim of this study was to study the prospective use of GAA in grossly bloody specimens and incorporate this in the flow of the laboratory.
Materials and Methods: All grossly bloody samples accessioned in the laboratory between 8 March, 2011 and 28 April, 2011, were processed using Hologic's ThinPrep® Pap Test protocol and Hologic's reprocessing protocol using 10% glacial acetic acid, without and with the addition of GAA. Both slides were reviewed by the cytotechnologists who signed out the normal cases, while the pathologist signed out the abnormal cases. All the cases were then reviewed by the pathologist and the slides were assessed for cellularity, cellular detail, presence of obscuring blood and abnormality. The overall unsat rate during the study period and the cause for an unsat PT, and the unsat rate for the previous 12 months were determined. An attempt was made to correlate them with the HPV test results (Invader assay performed without addition of GAA) and the histological follow-up. In addition, five known HPV positive and five known HPV negative cases were treated with GAA and reassessed using the Invader assay.
Results: Eighty cases were grossly bloody and were processed as two slides, with and without GAA, during the study period. There were 71 NILM, one other, three ASCUS, one ASCH, one AGUS, three LSIL, and no HSIL. One case was HPV positive and two were HPV negative. The unsat rate between May 2010 and April 2011, since the change in PT platform, had increased to 0.91%. Many of these were attributable to the use of a lubricant. Over the last six months the unsat rate was 0.67%. During the study period, the unsat rate reduced to 0.32%. When the two preparations were compared uniformly, all the slides with GAA showed a marked increase in cellularity, absence of obscuring blood, and improved cellular details.
Conclusions: GAA is useful in improving the cellularity of the bloody specimens. As it is considered ‘off-label’ to perform a GAA prep without first obtaining an UNSAT result using the Hologic's ThinPrep® Pap test protocol, it was determined that a GAA preparation was equivalent to Hologic's standard Pap Test protocol in this study. Although some of these specimens without GAA may not have been strictly considered unsat, addition of GAA vastly increased the cellularity, improved the cellular detail, and reduced the obscuring blood. Specimens with abundant blood could potentially be from patients with glandular lesions and may benefit from this process and manual screening instead of image-guided screening where they may be missed. Using GAA on grossly bloody specimens saves time upfront and can easily be incorporated into the work flow of the laboratory.








