Translate this page into:
Comparing endobronchial ultrasound-guided fine needle aspiration specimens with and without rapid on-site evaluation
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
We read with great interest the recent article by Carruth-Griffin et al. entitled “Utility of on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspiration specimens”[1] and commend the authors on a well-written paper, which addresses an important and timely topic of interest to cytologists evaluating these specimens. We would like to share our institution's experience as it relates to the use of rapid on-site evaluation (ROSE) in the clinical decision-making process for endobronchial ultrasound-guided fine needle aspiration (EBUS-FNA) cases, because our experience has been different and likely reflects different institutional practices.
With regard to clinical decision making at the time of ROSE, the impact of the ROSE may depend on who is performing the FNA. The authors of this recent study state that the EBUS-FNAs were performed mainly by clinicians/bronchoscopists in their Interventional Pulmonary Department.[1] In this setting, the patients are usually under conscious sedation in a bronchoscopy suite, and cannot have an immediate surgical intervention performed regardless of the ROSE. In our institution, the majority of the EBUS-FNA procedures are performed under general anesthesia in the operating room by thoracic surgeons. This is advantageous in that it allows for the patient to proceed to mediastinoscopy if the findings at the time of ROSE are benign or indeterminate, while sparing those patients with malignancy from having a more invasive procedure. The surgeons at our institution rely on the ROSE and preliminary diagnosis to help with their intraprocedural clinical decision making on whether or not to convert to a mediastinoscopy, which is similar to the use of intraoperative frozen section. The decision-making algorithm used at our institution is summarized in Figure 1 and has been previously published by our clinical colleagues.[2]
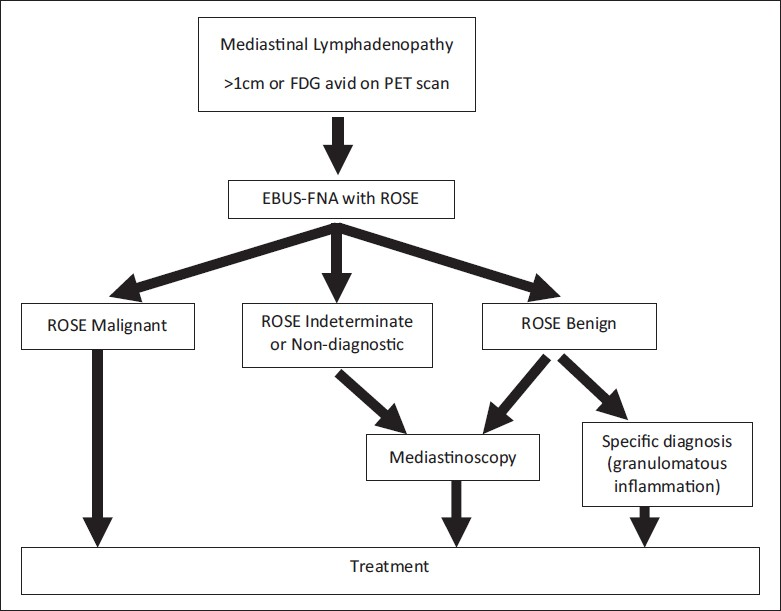
- Clinical decision-making algorithm for patients with suspicious mediastinal lymphadenopathy utilizing EBUS-FNA (FDG = Fluorodeoxyglucose, PET = Positron emission tomography, ROSE = Rapid on-site evaluation)
The paper also states that the diagnostic yield does not differ in cases with or without ROSE. However, in our experience, the use of ROSE allows one to evaluate for adequacy and to triage the material appropriately, which is particularly important for cases with a suspected malignancy where ancillary studies, including immunostains, flow cytometry, and/or molecular studies are extremely important.[3] Being present at the time of ROSE allows the cytologist to request additional material when needed and to allocate dedicated passes for cell block to enrich the cellularity, once a diagnosis can be reached. For instance, when present at the EBUS-FNA, if the first pass shows metastatic carcinoma, then any additional material from other passes can be used to make a cell block. However, in the absence of ROSE, the clinicians may use valuable material to make additional unnecessary slides, which could compromise the cell block yield and lead to the inability to perform ancillary studies, if these are to be performed on the cell block. Table 5 in the paper by Carruth-Griffin A et al.[1] points out that the number of cases with cell block preparation was slightly higher in the subset of cases with ROSE (92% vs 88%) and that more of the cases with ROSE had immunostains performed (29% vs 15%), special stains performed (9% vs 3%), and flow cytometry performed (11% vs 0.6%). Furthermore, the authors point out that although there was a similar percent of malignant cases in the cases with and without ROSE, immunostains were utilized more in those cases with ROSE (63% vs 37%), as was flow cytometry (94% vs 6%). The increased use of ancillary studies in the group with ROSE may reflect a better diagnostic yield of material for ancillary studies, in comparison with the group without ROSE. This is an important point because of the increasing need to perform molecular testing on non-small-cell carcinomas of the lung, which have predictive and prognostic value, and are becoming increasingly more important for the management of these patients. Given that cytological specimens are criticized for not having sufficient material for these important tests, we have found that being present to appropriately allocate sufficient material in EBUS-FNA cases for the aforementioned tests is important at our institution. After all, establishing a diagnosis of malignancy is just one aspect of what we are being asked to provide in today's era of personalized medicine. If we are unable to go further and perform crucial ancillary studies and molecular tests, then we are not optimizing the EBUS-FNA procedure and we are potentially subjecting a patient to additional diagnostic procedures, which increases healthcare costs and decreases the quality of care for a patient.[4]
In conclusion, the utility of ROSE in EBUS-FNAs may differ based on who is performing the procedure (clinician/bronchoscopist vs thoracic surgeon) and the different treatment algorithms employed in different institutions. This is important as many institutions are now trying to establish their own protocols for dealing with these new cytological specimens, which will likely increase over time as more minimally invasive approaches replace more invasive and costly surgical procedures. In our EBUS-FNA experience, if there is a potential for following the procedure with surgery, such as mediastinoscopy, then the use of ROSE with a preliminary diagnosis can be crucial for appropriate patient care and has a similar role as frozen section evaluation. As the results of this informative article point out, perhaps each institution should critically analyze their own practice to determine how best to use ROSE in EBUS-FNAs.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2012/9/1/2/92414
REFERENCES
- Utility of on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspiration specimens. Cytojournal. 2011;8:20.
- [Google Scholar]
- Should endobronchial ultrasonography be part of the thoracic surgeon's armamentarium? J Thorac Cardiovasc Surg. 2009;137:413-8.
- [Google Scholar]
- Diagnostic difficulties and pitfalls in rapid on-site evaluation of endobronchial ultrasound guided fine needle aspiration. Cytojournal. 2010;7:9.
- [Google Scholar]
- Seize the opportunity: underutilization of fine-needle aspiration biopsy to inform targeted cancer therapy decisions. Cancer. 2009;117:289-97.
- [Google Scholar]







