Translate this page into:
Comparison of liquid based cytology and conventional smears on lymph node aspirates: A cytomorphological study

*Corresponding author: Sana Ahuja, Assistant Professor, Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India. sanaahuja11@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Shahab J, Ahuja S, Singh M, Verma P, Ranga S. Comparison of liquid based cytology and conventional smears on lymph node aspirates: A cytomorphological study. CytoJournal 2024;21:7. doi: 10.25259/Cytojournal_22_2023
Abstract
Objective:
In an era of minimally invasive and rapid diagnostic technologies, fine-needle aspiration cytology (FNAC) is most useful when it comes to patients with lymphadenopathies especially of the cervical region. Liquid-based cytology (LBC) is an alternative processing method which is used for both gynecological and non-gynecological samples. Because of the remarkable advantages of LBC smears in gynecological samples, nowadays, many studies have been done to assess its utility in various other lesions. Hereby, with the help of this study, we would like to evaluate the efficiency of LBC smears in comparison to conventional FNAC smears conventional smears (CS) on lymph node aspirates.
Material and Methods:
A retrospective study was done over a 1-year period in which 253 cases of lymph node aspirates were included in the study. The slides were prepared using standard conventional and LBC techniques and compared for adequacy, cellularity, cell architecture, necrosis, background debris, presence of cells in monolayer sheets, and nuclear/cytoplasmic details.
Results:
Of the total 253 cases, 171 (67.6%) were and 67 (26.5%) were diagnosed as non-neoplastic and malignant, respectively. Although the LBC smears were useful in the diagnosis of malignant cases, they did pose some challenges especially in the non-neoplastic lymph node aspirates due to loss of the background necrosis. In addition, the cellular yield in LBC smears was low in comparison to CS.
Conclusion:
LBC smears from lymph node aspirates results in better diagnostic accuracy for malignant cases due to better cellular and nuclear details. However, for non-neoplastic etiology, it should not be considered better than CS as loss of the background necrosis and inflammation may result in an incorrect diagnosis.
Keywords
Liquid-based cytology
Conventional smears
Fine needle aspiration cytology
Lymph node aspirates
INTRODUCTION
Lymphadenopathies are most commonly caused by non-neoplastic etiology such as viral and bacterial infections. Other, less common causes include nodal accumulation of inflammatory cells in response to an infection in the node (lymphadenitis), neoplastic lymphocytes (lymphoma), or epithelial cells metastasizing to a lymph node (metastatic carcinomas) or storage diseases (Gaucher disease).
At times, it may become difficult for the physician to reach an accurate clinical diagnosis. Fine needle aspiration cytology (FNAC) is the investigation of choice for lymph node swellings since it is a minimally invasive, safe, and economical procedure which can be easily done on an outpatient basis to reach to a conclusive diagnosis.[1-3]
At the majority of medical centers that perform Fine needle aspiration cytology (FNAC), conventional smear (CS) cytology with alcohol fixation is the standard method for processing FNA specimens. Liquid-based cytology (LBC) is an alternative processing method in which the aspirated material is immediately fixed in either an ethanol or a methanol-based solution and is then placed on LBC slides. The LBC method is gaining popularity worldwide as the method of choice for not only gynecological but also for non-gynecological smears.[4-6]
LBC has many advantages such as rapid and proper fixation, reduced incidence of air-drying artifacts, cleaner background by hemolysis, even distribution of cells over a smaller slide area, and increased cellularity.[7,8] LBC allows the preservation of samples for some time and makes residual samples available for further investigations such as immunocytochemistry and even molecular analysis.[9,10,11] These advantages result in more objective diagnosis and greater diagnostic accuracy.[11]
Since there is scanty information available regarding the diagnostic value of LBC on lymph aspirates, the aim of the present study was to evaluate the efficiency of LBC smears in comparison to conventional FNAC smears (CS) on lymph node aspirates.
MATERIAL AND METHODS
A retrospective study was conducted over a period of 1 year from 2021 to 2022 on 253 patients with nodal swelling was easily visible with the naked eye. The different sites aspirated included 132 cases of cervical, 80 cases of axillary and 41 cases of inguinal swellings. The sample was processed using standard conventional and LBC techniques.
After clinical examination of swelling, FNAC was performed using a 23-gauge needle, 20-mL syringe, and syringe pistol without any ultrasound (USG) guidance. In the first step, air-dried and alcohol-fixed smears were made and stained with Giemsa and Papanicolaou stains, respectively. The remaining material left in the needle and syringe was transferred into 30 mL of CytoLyt solution (Cytyc) by rinsing the needle and syringe. It was centrifuged for 10 min at 500 rpm. The supernatant fluid was discarded and the material resuspended in a cryopreservative solution (PreservCyt, Cytyc). After 15 min, the material was processed in Becton Dickinson (BD) Surepath by standard operative procedure as described by the company. Immunocytochemistry (IHC) was also performed on the smears wherever needed, particularly in cases of metastatic carcinoma and lymphomas.
The CSs and LBC slides were examined without any knowledge of the diagnosis. The representative CSs and LBC slides were compared for adequacy, cellularity, background blood and cell debris, cell architecture, informative background (such as necrosis), presence of cells in monolayer sheets and nuclear/cytoplasmic details.
RESULTS
A total of 253 cases were diagnosed by LBC and conventional cytology [Table 1]. Majority cases were non-neoplastic (67.6%) comprising reactive lymphoid hyperplasia (107 cases, 42.3%), necrotizing granulomatous lymphadenitis (64 cases, 25.3%) [Figure 1]. Fifteen cases (5.9%) yielded an inadequate aspirate and were non-diagnostic. Out of all the malignant cases reported (67 cases, 26.5%), squamous cell carcinomas, adenocarcinomas (breast, lung, colon, and gallbladder), Hodgkin lymphomas and non-Hodgkin lymphomas accounted for 35 (52.2%), 12 (17.9%), 8 (11.9%), and 12 (17.9%) cases, respectively [Figure 2].
| S. No. | Cytological diagnosis | No. of cases | Percentage (%) | Cytomorphological findings in LBC |
|---|---|---|---|---|
| 1. | Reactive lymphadenitis | 107 | 42.3 | Florid mixed lymphoid population |
| 2. | Necrotising granulomatous lymphadenitis/Granulomatous lymphadenitis | 64 | 25.3 | Scant blotchy areas of necrosis and occasional epithelioid granulomas |
| 3. | Neoplasms | 67 | 26.5 | Metastatic squamous cell carcinomas, metastatic adenocarcinomas and lymphomas. Tumor cells arranged as monolayered sheets or scattered singly against a clearer background with better cellular details |
| 4. | Non diagnostic | 15 | 5.9 | Did not yield any material |
LBC: Liquid-based cytology
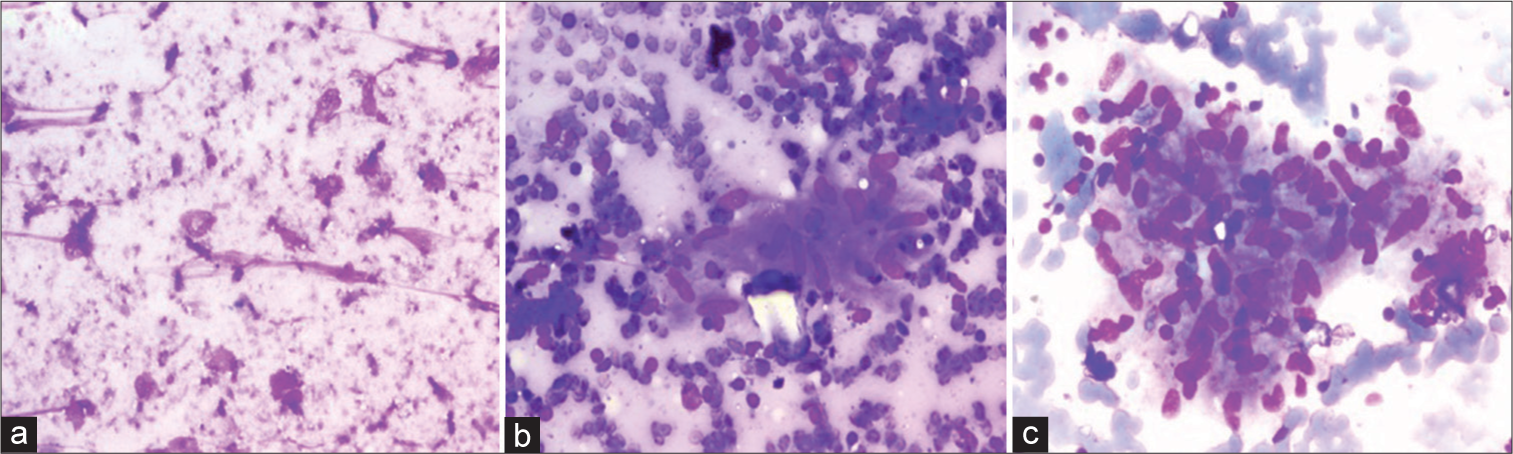
- A case of necrotizing granulomatous lymphadenitis on liquid-based cytology (LBC) and conventional smears (a and b) Giemsa stained sections show large areas of necrosis with epithelioid cell granulomas against a markedly hemorrhagic background on conventional smears (200×) (c) LBC slides show an epithelioid cell granuloma against a clean background with no necrosis and very few red blood cells (May Grunwald Giemsa, 200×).
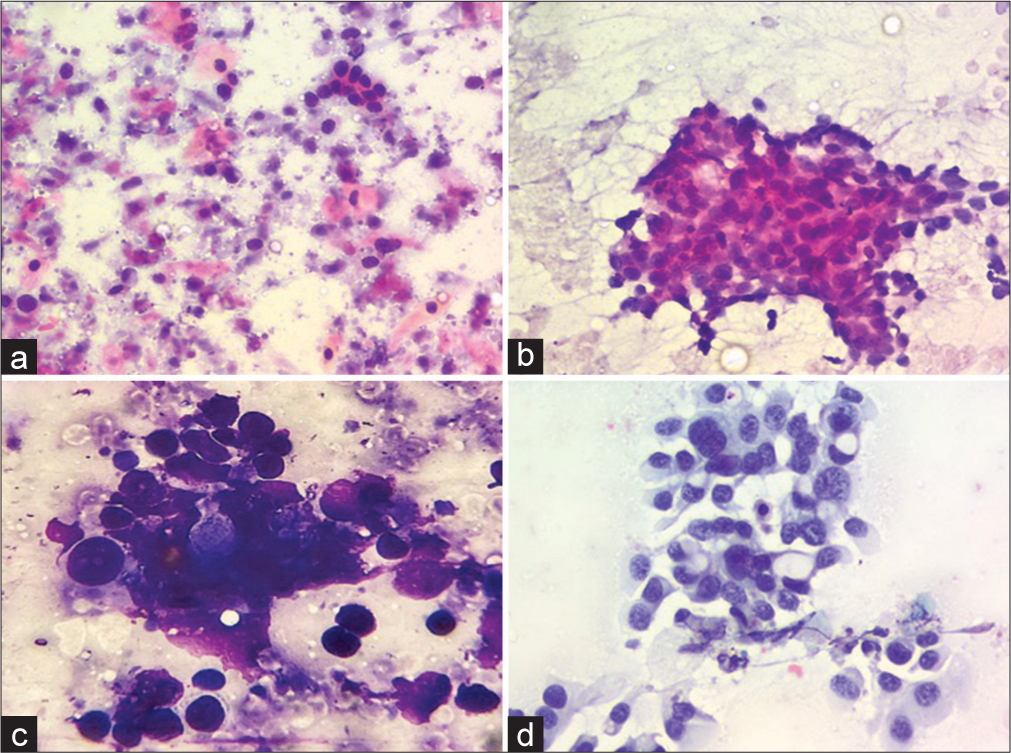
- Cytological features of malignant cases on liquid-based cytology (LBC) and conventional smears (a) A case of squamous cell carcinoma exhibiting atypical cells scattered against a background with tumor diathesis on conventional Giemsa stained smears (200×) (b) The same case exhibiting monolayered sheet of tumor cells with better nuclear details against a clear background on LBC Papanicolaou stain (200×) (c) A case of metastatic adenocarcinoma exhibiting a cluster of atypical cells with nuclear overlapping and overcrowding with obscured nuclear details on conventional Giemsa stained smears (400×) (d) The same case exhibiting monolayered fragment of tumor cells with clear nuclear details with conspicuous nucleoli against a clean background on LBC (200×, Papanicolaou stain ).
Although the LBC smears were useful in the diagnosis of malignant cases, they did pose some challenges especially in the non-neoplastic lymph node aspirates due to loss of the background necrosis. In addition, the cellular yield in LBC smears was low in comparison to CS. The differences in the conventional and LBC slides have been tabulated in Table 2.
| S. No. | Cytological diagnosis | Cytological features on CSs | Cytological features on LBC |
|---|---|---|---|
| 1. | Reactive lymphadenitis | Florid mixed maturing population of lymphoid cells against a hemorrhagic background | Clear background showing only maturing lymphoid cells with no blood in the background |
| 2. | Necrotizing granulomatous lymphadenitis | Epithelioid cell granulomas and Langhans giant cells against large areas of necrosis in the background | Clearer background, occasional scattered epithelioid cell granulomas and giant cells. No necrosis in the background |
| 3. | Neoplasms | Cytomorphological features and arrangements are easily appreciated on CSs. However, obscuration by blood or tumor diathesis may result in difficult interpretation | Nuclear details are better appreciated on LBC due to a cleaner background and monolayered sheets. In addition, it is easier to perform ancillary techniques like immunocytochemistry on LBC slides |
LBC: Liquid-based cytology, CS: Conventional smear
DISCUSSION
LBC has gained great success in recent years in gynecological cases. However, the cellularity in LBC and conventional cytology smears are similar but the nuclear details are clearer in LBC.[12-15] There are almost no red blood cells in the background in comparison to the CSs where hemorrhagic background creates a problem in the final impression in all the cases.[14] The LBC was very useful in diagnosis of malignant lesions like metastatic carcinomas and lymphomas since it enhanced the nuclear details and architectural features which made it easier to arrive at the final diagnosis. However, compared to conventional cytology, in LBC, the background material was washed off which was a major drawback, since the background material like necrosis and mucin did provide information about the nature of the lesions and aids in its final diagnosis. Furthermore, this background material helps in arriving at the final diagnosis in non-malignant lymph node aspirates when examined in CSs.
After making a cytological diagnosis, whatever material remaining in the vial can be used for the application of ancillary techniques such as immunocytochemistry (ICC), flow cytometry, and molecular biology because the LBC method enables the storage of a variable number of cells for up to 6 months after the FNAC.[6,7,14-15]
Most of the causes of lymphadenopathy include reactive hyperplasia, granulomas, and metastatic cancers as well as lymphoma. The cases of lymphoma can be accurately diagnosed based on cytomorphological findings on Giemsa-stained smears as well as flow cytometric.
Rapid onsite evaluation is very critical before recommending ancillary studies to ensure an optimal cell block. The diagnostic yield of FNA when done with flow cytometry and the cell block is comparable to that of core needle biopsy. FNA is a minimally invasive technique with a shorter turnaround time and provides a content rich sample for flow cytometry. A dedicated pass should be done for immunophenotyping/ cell blocking in cases where clinical suspicion of lymphoma is high.[16]
The problems associated with immunocytochemistry include difficulty in evaluation of coordinate immunoreactivity patterns. This is based on the evaluation and correlation of exactly the same cells and microtissue fragments which is not possible in immunocytochemistry slides. In addition, the interference due to exposure to fixatives and reagents during processing of cytology preparations is another drawback.[16]
In lymph node FNAC, the air-dried smears (Giemsa stained) show greater cellularity and less erythrocyte interference as compared to the alcohol fixed smears. Furthermore, the air-dried smears stored up to 72 h on staining with hematoxylin and eosin and Papanicolaou stain showed comparable cytomorphology to fresh smears.
In a study conducted by Bandoh et al.,[15] cell types and cellular arrangements were well preserved in LBC slides and a higher diagnostic accuracy was seen than with CSs.[15] Dey et al.[14] concluded that it is easier and less time consuming to interpret LBC slides as the cells are spread over a smaller area as monolayered sheets with a clearer background. However, LBC preparations are more expensive and require some experience for interpretation.[14] Erdogan-Durmus in their study concluded a comparable diagnostic accuracy between the two techniques.[1]
CONCLUSION
LBC cannot be implemented as an alternative to conventional cytology; however, it is a good adjunct since it provides excellent cellular details including better nuclear features. In addition, the limitations of conventional cytology such as obscuring background and drying artifacts are minimized. It also minimizes the need for repeat aspiration by utilizing the residual material. However, the major drawback of LBC is that it clears the background material. This may result in loss of mucinous/necrotic background, which may be very crucial for diagnosis of necrotizing lymphadenitis cases and certain malignancies.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ABBREVIATIONS
CS - Conventional smear
FNAC - Fine needle aspiration cytology
ICC – Immunocytochemistry
LBC - Liquid based cytology
USG - Ultrasound.
AUTHOR CONTRIBUTIONS
SA: Conceptualization, data curation, formal analysis, methodology, writing: review and editing; JS: Data curation, writing original draft; MS: Conceptualization, data curation, formal analysis, supervision; PV: Data curation, formal analysis; SR: Data curation, resources, supervision. Each author has participated sufficiently in the work and takes responsibility for appropriate portions of the content of this article. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was exempted from approval from the Institutional Ethics Committee due to its retrospective nature and use of anonymised data.
Written informed consent was obtained from all the participants prior to the publication of this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
None.
References
- Diagnostic value of liquid-based cytology test in intrathoracic lymph nodes and lung lesions sampled by endobronchial ultrasonography-transbronchial needle aspiration. Diagn Cytopathol. 2021;49:1251-6.
- [CrossRef] [PubMed] [Google Scholar]
- Liquid-based preparations versus conventional cytology: Specimen adequacy and diagnostic agreement in oral lesions. Med Oral Patol Oral Cir Bucal. 2005;10:115-22.
- [Google Scholar]
- Comparison of TriPath thin-layer technology with conventional methods on nongynecologic specimens. Acta Cytol. 2000;44:567-75.
- [CrossRef] [PubMed] [Google Scholar]
- Significance of node biopsy before definitive treatment of cervical metastatic carcinoma. Laryngoscope. 1978;88:594-7.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound guided core biopsy, fine needle aspiration cytology and surgical excision biopsy in the diagnosis of metastatic squamous cell carcinoma in the head and neck: An eleven year experience. Eur J Radiol. 2010;80:792-5.
- [CrossRef] [PubMed] [Google Scholar]
- Management of lymph node metastases from an unknown primary site to the head and neck (Review) Mol Clin Oncol. 2014;2:917-22.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of liquid-based cytology versus conventional smears in FNA samples. J Cytol. 2015;32:17-20.
- [CrossRef] [PubMed] [Google Scholar]
- The potential of liquid-based cytology in lymph node cytological evaluation: The role of morphology and the aid of ancillary techniques. Cytopathology. 2016;27:50-8.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnosis of non-Hodgkin lymphoma using fine-needle aspiration cytopathology: A work in progress. Cancer Cytopathol. 2010;118:238-43.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of liquid-based (Thinprep) preparations in nongynecologic cases. Diagn Cytopathol. 2001;24:137-41.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration in the diagnosis of head and neck lesions: A review and discussion of problems in differential diagnosis. Diagn Cytopathol. 2007;35:798-805.
- [CrossRef] [PubMed] [Google Scholar]
- Reliability of fine needle aspiration cytology (FNAC) as a diagnostic tool in cases of cervical lymphadenopathy. J Egypt Natl Canc Inst. 2011;23:105-14.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of ThinPrep and conventional preparations on fine needle aspiration cytology material. Acta Cytol. 2000;44:46-50.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of liquid-based cytology with fine needle aspiration specimens for cervical lymphadenopathy. Diagn Cytopathol. 2016;44:169-76.
- [CrossRef] [PubMed] [Google Scholar]
- Routine air drying of all smears prepared during fine needle aspiration and intraoperative cytology studies. An opportunity to practice a unified protocol offering the flexibility of choosing a variety of staining methods. Acta Cytol. 2001;45:60-8.
- [CrossRef] [PubMed] [Google Scholar]








