Translate this page into:
Cytokeratin 20/p53 dual immunocytochemistry for improving the diagnostic accuracy of urine liquid-based cytology in the detection of urothelial neoplasm: A retrospective study
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Backgrounds:
Dual immunocytochemistry (DIC) with cytokeratin (CK) 20 and p53 in liquid-based cytology is a tool for improving the accuracy of urine cytology (UC). This study was conducted to compare the diagnostic accuracy of UC alone with that of UC combined with CK20/p53 DIC.
Methods:
We retrieved urine samples collected between January 2015 and March 2016 stored in PreservCyt®solution that were from cases categorized as malignant, highly suspicious, suspicious, and atypical and that were matched with a subsequent biopsy. We re-prepared 63 samples of 28 patients for DIC and blindly evaluated 63 pairs of original Papanicolaou smears and DIC.
Results:
Of the 63 samples, 11 could not be analyzed because of the low number of atypical urothelial cells, and the results of the remaining 52 samples were as follows: 34 positive and 18 negative. The positive predictive value of DIC was 100%, and the negative predictive value was 78%. Fifteen DIC-positive cases, histologically proven as malignant were originally diagnosed as highly suspicious (4), suspicious (8), and atypical (3), which were strongly suggestive of “urothelial carcinoma”. Four negative cases, histologically confirmed as non-neoplastic cases, were filtered from false positivity.
Conclusions:
Despite the small sample size, this study demonstrated the diagnostic utility, high sensitivity, and positive predictive value of CK20/p53 DIC, especially in cases with a small number of single malignant cells or cellular clusters of reactive atypical urothelial cells. Thus, CK20/p53 DIC can be used for improving diagnostic accuracy of UC, either as an ancillary method to cytology or as a part of a potential future diagnostic panel to improve patient diagnosis and management.
Keywords
Cytokeratin 20
dual immunocytochemical staining
p53
urothelial carcinoma
BACKGROUND
The vast majority of all urothelial carcinomas are bladder urothelial carcinoma (BUC), which is the most common malignancy of the urinary tract.[1] Approximately 75-85% of the patients with BUC present with non-muscle invasive BUC, at stage Ta, Tis, or T1.[2] Even when the optimal treatment modality is selected and administered, approximately, 70% of cases of BUC recur, and progression has been reported in 30% of cases. Early detection of primary or recurrent neoplasm facilitates timely management, resulting in better clinical outcome.[2] Thus, accurate diagnostic modalities play a crucial role in improving the treatment outcomes of BUC.
Urine cytology (UC) in conjunction with cystoscopy is the “gold standard” for BUC screening and follow-up, but the cystoscopy procedure is associated with patient-discomfort and stress.[3] The median sensitivity and specificity of UC alone have been reported as 35% and 94%, respectively. However, its sensitivity in low-grade tumors is as low as 17% compared with its 58% sensitivity in high-grade tumors.[2] Interobserver variability and variability in their expertise of the cytopathologists further reduce the reproducibility of urinary cytology.[4]
Therefore, several additional urinary cytological assessments have been developed over the last 20 years to improve the sensitivity of UC detection rate. One of these tests is fluorescence – in situ hybridization (UroVyison®)[5] for the detection of chromosomal aberrations; loss of the 9p21 locus, which is the site of the p16 tumor suppressor gene, has been identified as a common chromosomal aberration in patients with UC. However, the pitfall of FISH test is chromosomal abnormalities even in benign cells. The final conclusion is suggested that FISH analysis should be performed by persons with experience in cytopathology and not be delegated to purely technical staff.[5] Another newly developed test is an immunocytology test (uCyt + test[6]), which combines conventional UC with an immunofluorescence assay using a cocktail of antibodies against UC-associated surface antigens such as carcinoembryonic antigen and mucin-like glycoprotein. Furthermore, protein-based tests such as the quantitative nuclear matrix protein 22-enzyme-linked immunosorbent assay (NMP22-ELISA[7]) are widely available and frequently used by specialized centers and practitioners. A common limitation of some these markers is the relatively high rate of false-positive results due to endogenous or exogenous factors that include mechanical manipulations, urinary tract infection, hematuria, and impaired renal excretory function. Some investigators have investigated the use of a combination of the four available urine tests (CYT, FISH, uCyt+, and NMP22-ELISA) in a large cohort of 808 patients with suspected BUC.[8] A comparative analysis of diagnostic tests for urothelial carcinoma: Cxbladder detect, UroVysion® FISH, NMP22, and cytology had performed based on imputation of multiple datasets.[9] The methodology was validated for comparative ranking of the diagnostic tests for detecting BUC.
Dual immunocytochemistry (DIC) with p53 and cytokeratin (CK) 20, in liquid-based cytology (LBC), is a tool for improving the accuracy of UC. CK20 is normally expressed only in umbrella cells, and dysregulation of CK20 is an early event of carcinogenesis. Abnormal expression beyond superficial layers has been used as a marker for the diagnosis of urothelial carcinoma.
P53 is a tumor suppressor gene, playing a role in apoptosis, genetic stability, and inhibition of angiogenesis. Mutation of the p53 gene prolongs the half-life of the protein resulting in nuclear p53 accumulation, which is detectable by immunohistochemistry.
Recently we performed directly DIC in LBC urine sample, which yielded a satisfactory result for the detection of urothelial carcinoma.[10] Therefore, we conducted this study to evaluate the diagnostic utility of DIC for improving the specificity and positive predictive value of UC.
METHODS
A search of the records of pathology department of Inje University, Sanggye Paik Hospital revealed 139 urine samples with matching histology, collected between January 2015 and March 2016. During that period, 63 samples of residual ThinPrep® (Hologic, Inc. Marlborough, MA, USA) material collected from 28 patients were stored in the refrigerator for further studies, that the original cytologic diagnoses were 25 malignant, 6 highly suspicious, 13 suspicious, and 18 atypical. Subsequently, a histology data search was performed because the urine samples with “negative” samples were discarded after the 3 months; the retained sample was more than “atypical”. We re-prepared urine samples stored in PreservCyt®solution for immunocytochemical staining.
Immunocytochemical analysis was performed by automated platform (Leica, Bond®; Vision Biosystems, Melbourne, Australia). CK20 (monoclonal mouse, clone Ks 20.8; Diagnostic Biosystems, Pleasanton, CA, USA) and p53 (monoclonal mouse, clone DO-7; Dako A/S, Glostrup, Denmark) antibodies were used for DIC. The DIC protocol comprised antigen retrieval through pretreatment with citrate-based buffer pH 6.0 at a high temperature, endogenous peroxidase activity blocking with hydrogen peroxide for 10 min and finally incubation with CK20 antibody (dilution 1:50) for 18 min. Subsequently, the slides were treated with post-primary IgG linker reagent for 10 min followed by poly-HRP IgG reagent for another 10 min. After exposure to mixed 3, 3’ -diaminobenzidine-Refine for 7 min before incubation with the second primary antibody anti-p53 antibody (dilution 1:100) for 15 min. Post-primary IgG linker was applied again to the slides for 20 minutes, followed by poly-AP IgG reagent for 30 minutes and then mixed red-refine for 15 min. Finally, the slides were counterstained with Harris hematoxylin for 5 min, dehydrated with graded alcohol, cleared in xylene and mounted in dibutylphthalate polystyrene xylene. The slides were rinsed well with distilled water and wash buffer between all steps.
Brown nuclear staining was considered positive for p53 and red cytoplasmic staining for CK20. Superficial umbrella cells were served as internal positive controls for CK20. [Figure 1] CK20 weak staining was considered as a negative result. Immunocytochemical staining was considered positive only when present in atypical cells.
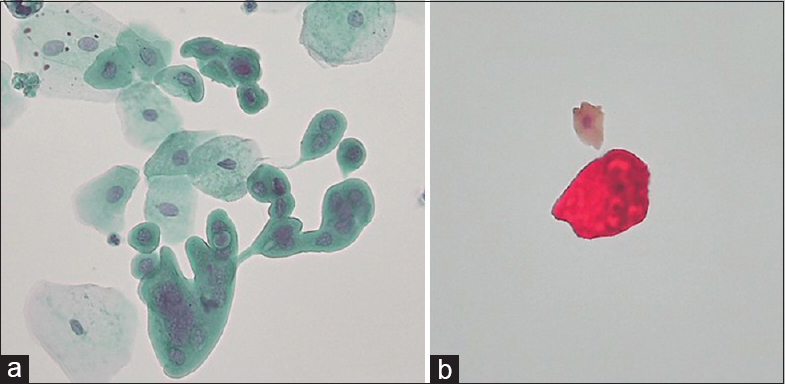
- (a) Papanicolaou smear of urine cytology sample showing atypical cells with multinucleation and vacuolated cytoplasm indicates umbrella cells. (b) Dual immunocytochemistry smear showing cytoplasmic red staining (CK20) without nuclear brown expression (p53) supports of umbrella cells.
RESULTS
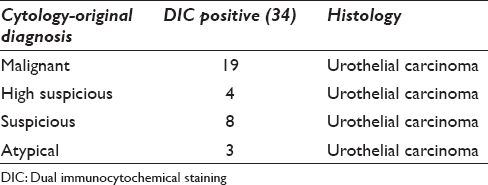
Fifty-two out of 63 cases were evaluated. Eleven samples were not available because of the paucity or absence of atypical cells. Both CK20 and p53 were expressed in 34 samples, which were histologically confirmed as urothelial carcinoma. Thus, DIC had a positive predictive value of 100. The original cytological diagnoses were malignant (19), highly suspicious (4), suspicious (8), and atypical (3) [Table 1]. Two out of three atypical cases showed low population of tumor cells. One case showed degenerated cytomorphology in the bloody background, in which distinct CK20/p53 double staining of the tumor cells was visible [Figure 2]. CK20/p53 double positivity was readily apparent in the other cases.
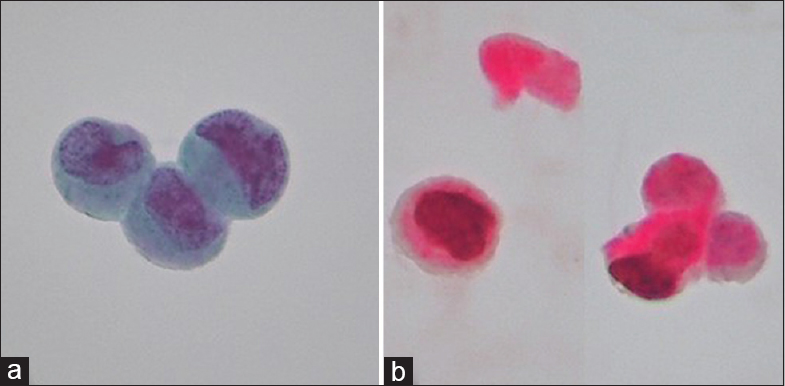
- (a) A urine cytology sample showing a few clusters of atypical cells (Original cytologic diagnosis; suspicious) is seen. (b) Dual immunocytochemistry smear revealed cytoplasmic red (CK20) and nuclear brown (p53) indicates urothelial carcinoma cells.
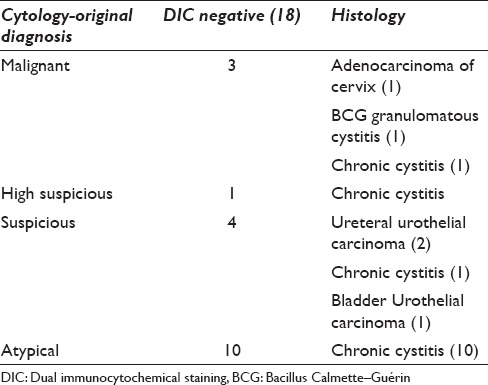
Eighteen cases were not positive for both markers. Fourteen out of eighteen cases were histologically unrelated to urothelial carcinoma, yielding a negative predictive value of 78%. The original cytological diagnoses were malignant (3), highly suspicious (1), suspicious (4), and atypical (10) [Table 2]. Two cases of cystic and 2 cases of ureteral urothelial carcinomas were negative for CK20/p53 double staining. One case of bladder tumor showed many clusters with p53 nuclear staining [Figure 3]. Thirteen cases of chronic cystitis can be avoided from false positivity.

- (a) A urine cytology sample showing a few degenerated atypical cells (original cytologic diagnosis; highly suspicious). (b) Dual immunocytochemistry revealed CK20 only, histologically confirmed as bacillus Calmette–Guérin granulomatous cystitis.
Thus, the CK20/p53 DIC yielded in a sensitivity of 89%, specificity of 100%, positive predictive value of 100% and negative predictive value of 78% [Table 3]. Because cases other than those with only atypical cytology were included in this study (atypical, suspicious, highly suspicious, and malignant) the diagnostic accuracy of the cytology could not directly compared with that of DIC. The positive predictive value of cytology alone was 73% vs. 100% for UC combined DIC.

DISCUSSION
Current guidelines recommend performing UC as an adjunctive tool in patients with suspected UC.[3] Although UC has relatively high specificity, its diagnostic value is impaired because of its low sensitivity.[2] Therefore, several additional urinary cytological assessments have been developed over the last 20 years to improve the detection rate of UC. Multiprobe Fluorescence in situ hybridization (UroVysion) has been most commonly used and especially indicated for (1) atypical urinary cytology (2) control after intravesical BCG treatment (3) upper urinary tract cytology (4) surveillance after transurethral resection and 5) hematuria inpatients with an increased risk of UC.[5] However, this method is time-consuming and requires trained personnel. Furthermore, the high cost makes. difficult to perform routinely.
Another group of investigators designed a study to investigate the use of a triple cocktail of antibodies against CK20, p53, and CD44 in urine cytology samples for identifying and differentiating UC from benign mimics.[11] This triple cocktail method had been tried on tissue specimens. Triple immunostaining of sections of.urinary cytological specimens imbedded in paraffin yielded 94% sensitivity and 93% specificity for UC.[12] They reported that CK20 and p53 were useful for identifying cases with subsequent carcinoma. CD44 was very reliable, being present only in a minority of cases.
Because the LBC urine samples had been collected starting from January 2015 with atypical cases according to the original cytologic diagnosis, the number of sample retrieved was limited. Among them, only some had been histologically confirmed. Eleven cases could not be analyzed because of the extremely low number of urothelial cells.
CK20/p53 DIC has been recently studied in another study group.[13] They classified the samples into positive and negative groups on the basis of UC. A comparison of the accuracy of cytologic diagnosis with that of cytology with immunocytochemical staining revealed the following results: sensitivity 73.4% vs. 91.1%, specificity 100% vs. 74.3%, positive predictive value 100% vs. 88.9%, and negative predictive value 62.5% vs. 78.8%, respectively. Their results showed that DIC improved the sensitivity of UC. In contrast, our results showed that DIC improved specificity and positive predictive value. The difference is caused slikely by differences in that diagnostic threshold and the collecting method between the two studies.
The use of other marker combination such as p53/Ki-67 has been proposed for improving urothelial carcinoma detection in UC.[14] However in that study, p53/Ki-67 DIC and UC were not performed on the same slide, but on separate Cytospine samples. Therefore, the immunophenotypic features of the same atypical cells could not be determined.
Thus, in this study, DIC ensured the detection of urothelial carcinoma in cases with vague cytological diagnoses (atypical, suspicious, highly suspicious) and made it possible to distinguish these cases from benign (reactive) atypia.
CONCLUSIONS
Despite the small sample size, this study demonstrated the diagnostic utility with high sensitivity and positive predictive value of CK20/p53 DIC, especially in cases with a small number of malignant cells or cellular clusters of reactive atypical urothelial cells. A proper indication of bordered cases between reactive and malignant would be helpful for differentiation. Therefore, our results strongly suggest the use of CK20/p53 DIC for improving the diagnostic accuracy of UC. It may be used either as an ancillary method to cytology or as part of a potential future diagnostic panel to improve patient diagnosis and management.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
HK designed this study. JY contributed a urine sample and tissue specimen with informed consent and collaborated on ancillary studies using liquid-based smear (urine). All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study was approved from Institute Review Board of Inje University, Sanggye Paik Hospital Acknowledgments (IRB number; 2016-08-033; date: August 31, 2016).
LIST OF ABBREVIATIONS (In alphabetic order)
BUC - Bladder urothelial carcinoma
CK - Cytokeratin
DIC - Dual immunocytochemical staining
LBC - Liquid-based cytology
UC - Urine cytology.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model. (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENT
This work is supported by the Inje University Research Foundation (20110767). This study was presented in the form of a poster (poster #16) at the 30th annual spring meeting of the Korean Society for Cytopathology.
REFERENCES
- European Association of Urology. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur Urol. 2013;64:639-53.
- [Google Scholar]
- Diagnostics techniques in nonmuscle invasive bladder cancer. Indian J Urol. 2015;31:283-8.
- [Google Scholar]
- The value of urine cytology in the diagnosis of bladder cancer. Cytopathological correlation. Saudi Med J. 2013;34:937-41.
- [Google Scholar]
- Immunocytology is a strong predictor of bladder cancer presence in patients with painless hematuria: A multicentre study. Eur Urol. 2012;61:185-92.
- [Google Scholar]
- Multiprobe fluorescence in situ hybridization (Uro Vysion) for the detection of urothelial carcinoma-FISHing for the right catch. Acta Cytol. 2011;55:113-9.
- [Google Scholar]
- Immunocyt: A new tool for detection in voided urine samples. J Urol. 1999;161:1486-9.
- [Google Scholar]
- Systemic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess. 2010;14:1.
- [Google Scholar]
- Combined application of cytology and molecular Urine markers to improve to detection of Urothelial carcinoma. Cancer Cytopathol. 2013;121:252-60.
- [Google Scholar]
- A holistic comparative analysis of diagnostic tests for urothelial carcinoma: A study of Cxbladder detect, UroVysion ® FISH, NMP22® and cytology based on imputation of multiple datasets. BMC Medical Research Methodology. 2015;15:45.
- [Google Scholar]
- Diagnostic utility of double immunostaining of a urine cytology preparation for cytokeratin 20/p53 expression in a young woman with micropapillary urothelial carcinoma of the renal pelvis presenting as an unknown primary malignancy. Cytojournal. 2016;16(13):26.
- [Google Scholar]
- Discriminatory immunohistochemical staining of urothelial carcinoma in situ and non-neoplastic urothelium: An analysis of cytokeratin 20, p53, CD44 antigens. Am J Surg Pathol. 2001;25:1074-8.
- [Google Scholar]
- Evaluation of a triple combination of cytokeratin 20, p53, CD44 for improving detection of urothelial carcinoma in urine cytology specimens. CytoJournal. 2013;10:25.
- [Google Scholar]
- Evaluation of double immunocytochemical staining for CK20 and P53 as a potential adjunct to cytology for urothelial cancer diagnosis. Cytopathology. 2017;28:96-102.
- [Google Scholar]
- Immunocytochemical staining for p53 and Ki-67 helps to characterize urothelial cells in urine cytology. Cytopathology. 2016;27:456-64.
- [Google Scholar]








