Translate this page into:
Cytologic features of primary monophasic synovial sarcoma of the thyroid gland
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Synovial sarcoma (SS) is a rare soft tissue tumor, commonly arising in para-articular areas of extremities, but can also present in the head and neck area. However, primary SS of the thyroid gland is an extremely rare tumor which has been reported only five times in previous English literatures. This report presents fine needle aspiration (FNA) cytology of primary monophasic SS of the thyroid gland. A 47-year- old woman incidentally detected thyroid nodule in the isthmus of right thyroid gland on an ultrasonography by regular health check-up. Because the possibility of malignancy could not be ruled out, FNA and surgical resection were performed. The cytological, histopathological, immunohistochemical, and molecular genetic study of SYT-SSX transcript were discussed. For the past 3 years of follow-up after surgery, no recurrence or metastasis has been identified.
Keywords
Synovial sarcoma
SYT-SSX transcript
thyroid gland
INTRODUCTION
Synovial sarcoma (SS) is known as soft tissue tumor, commonly arising in para-articular areas of extremities. However, it can also occur in the head and neck area including thyroid gland. Of the tumors in the head and neck area, SS accounts for about 9% of all cases.[1] However, primary SS of the thyroid gland is an extremely rare tumor which has been reported only five times in previous English literature.[23456] Here, we present a patient with primary monophasic SS (MSS) of the thyroid gland and its cytologic, histopathologic, immunohistochemical, molecular analytic findings.
CASE REPORT
A 47-year-old woman incidentally detected thyroid mass on regular health check up. There was no history of swallowing difficulty or weight loss. Physical examination and laboratory findings including thyroid function test were unremarkable. On the thyroid ultrasonography, 7.8mm sized anechoic lesion suspected of malignancy was found in the isthmus of right lobe. No significantly enlarged cervical lymphadenopathy was noted [Figure 1].

- Ultrasonography of the thyroid gland with 7.8 mm anechoic lesion in the isthmus of right lobe
The patient underwent ultrasonography-guided fine-needle aspiration (FNA). The overall cytologic feature showed large quantity of either single or clustered ovoid to spindle cells [Figure 2a]. Some inflammatory cells such as lymphocytes, neutrophils, eosinophils, and plasma cells were seen in a bloody background. The tumor cells formed small and large three-dimensional compact clusters with ragged edges [Figure 2b]. The nuclei of these cells were monotonous, oval to spindle shaped, and rich in fine granular chromatin with inconspicuous nucleoli. The cytoplasm was scanty and pale, making the nuclei resemble naked nuclei [Figure 3a]. The cytologic features of single cells were similar to those of cluster-forming cells, suggesting that single cells had become detached from clusters [Figure 3b]. Atypical cells with acinar or gland forming structures, mitosis, and necrosis were not observed. These cytologic findings could not provide a definitive diagnosis of whether the lesion is benign or malignant.

- (a) The cytologic findings revealed a hemorrhagic background with high cellularity which is composed of variable-sized nests and scattered naked cells (H and E, ×40). (b) The ovoid to spindle tumor cells form clusters with storiform arrangement (H and E, ×100)
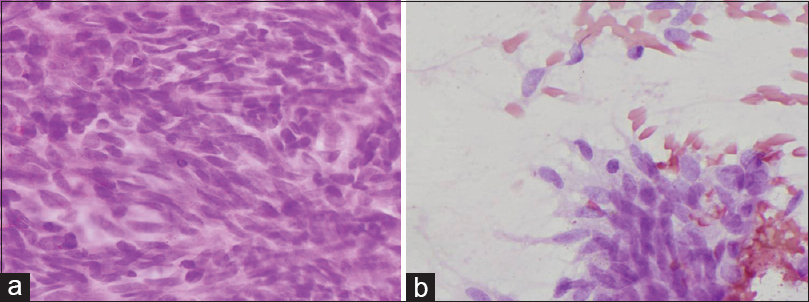
- (a) Monotonous oval to spindle shaped nuclei with fine granular chromatin, inconspicuous nucleoli and scant cytoplasm (H and E, ×400). (b) The scattered cells similar to clustered tumor cells (H and E, ×400)
Subsequently, the patient underwent a total thyroidectomy. Grossly, the thyroid mass was a solid lesion with the greatest dimension of 0.8 cm and revealed irregular shape with no capsule [Figure 4a]. The surface was grayish white, and there was some hemorrhage but no necrosis. Microscopically, the tumor was composed of ovoid to spindle tumor cells, forming fascicles with an interlacing arrangement [Figure 4b]. The cells showed ovoid to spindle nuclei with fine chromatin and inconspicuous nucleoli that were similar to the cytologic findings as mentioned above. No acinar or glandular component was observed. Some inflammatory cells such as lymphocytes and eosinophils were admixed with tumor cells [Figure 4c and 4d]. There was no collagen, amyloid-like materials, or mitosis.

- (a) 0.8 cm sized high cellular tumor without capulation (circle, H and E, ×20). (b) Well-demarcated mass from normal thyroid tissue (H and E, ×40). (c) Histopathology of mass in thyroid gland showing spindle-shaped tumor cells with storiform and admixed with various inflammatory cells (H and E, ×100). (d) Fine granular nuclei with inconspicuous nucleoli and scant cytoplasm (H and E, ×400)
Immunohistochemistry exhibited the positive reactivity for epithelial membrane antigen [Figure 5a], pancytokeratin (CK) [Figure 5b], vimentin [Figure 5c], and transduction-like enhancing protein 1 [Figure 5d]. The expression of neuroendocrine markers, CD56, synaptophysin, and chromogranin, was not found. The tumor cells also showed negative reactivity for calcitonin, CD34, thyroid transcription factor-1, S-100, c-kit for differentiating from the other spindle cell tumors. Furthermore, fluorescence in situ hybridization using paraffin block demonstrated translocation (X; 18)(p11;q11). Consequently, the diagnosis was made as SS [Figure 6]. Additional whole-body positron emission tomography-computed tomography was performed to determine the origin of MSS. There was no other mass or metastasis. For the past 3 years of follow-up after surgery, no recurrence or metastasis has been identified.
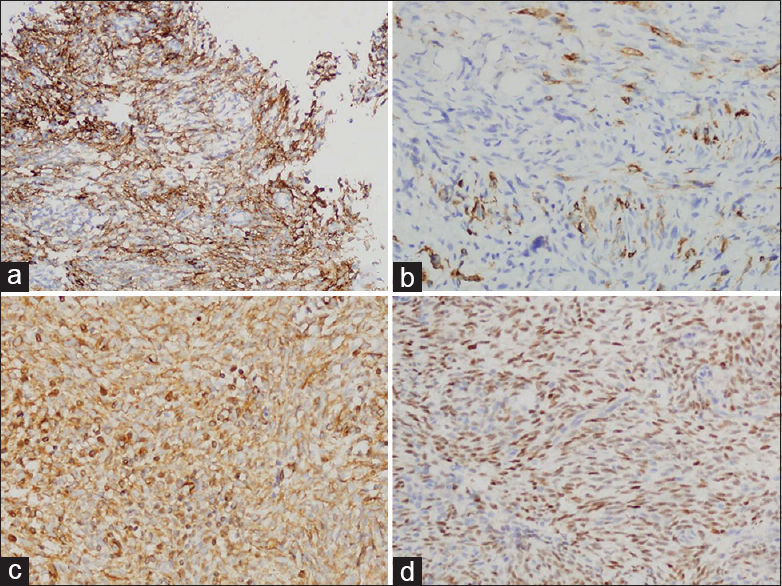
- Immunohistochemical expression of EMA (a), CK (b), vimentin (c), and transduction-like enhancing protein 1 (d) in tumor cells

- Fluorescence in situ hybridization showing t (X; 18) using a dual-color break-apart probe. The 3’ probe proximal to the SYT gene (green) and the 5’ distal probe (red) are separated in abnormal cells expressing the translocation
DISCUSSION
FNA is easy to apply, has low complication rates and high diagnostic value, and is a cost-effective test used in the diagnosis of thyroid nodules. Currently, FNA is the preferred diagnostic method for the initial stage of evaluation of thyroid nodules.[7]
SS is a malignant mesenchymal tumor, commonly arising in para-articular areas of lower extremities. It is thought to originate from multipotent stem cells, not from synovium; therefore, it can occur at any sites.[8] SSs arising in head and neck account for about 9% of all SS.[1] However, SS arising in the thyroid gland is extremely rare and they have been reported only five times in previous English literature.[23456] Kikuchi et al. first reported SS of the thyroid gland in 2002. Previously reported clinical and histologic findings are summarized in Table 1.
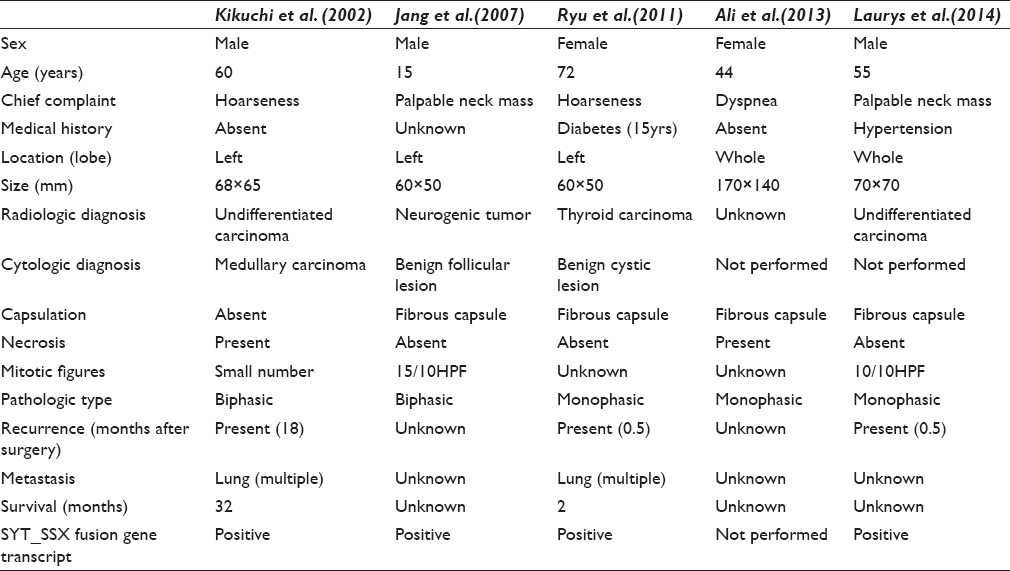
SSs are classified into two main subtypes; monophasic and biphasic. MSS is characterized by bipolar or fusiform nuclei with fine granular chromatin and scant cytoplasm.[9] Our case showed spindle cells without atypia. However, mitosis, necrosis, and capsulation were not observed unlike the previously reported cases [Table 1]. These cytologic features need to be distinguished from the following diseases; medullary carcinoma, spindle epithelial tumor with thymus-like differentiation (SETTLE), undifferentiated carcinoma, ectopic hamartomatous thymoma, fibroma, low-grade fibrosarcoma, malignant peripheral nerve sheath tumor (MPNST), and other mesenchymal tumors. The characteristic cytologic features of medullary carcinoma are polygonal and spindle cells with fine chromatin and inconspicuous nucleoli, which makes it difficult to distinguish between medullary carcinoma and MSS. However, 44%-64% of medullary carcinomas have amyloid and less polymorphism than SS.[2] SETTLE may show ovoid to spindle cell nest and single cells like MSS, but often biphasic.[10] Undifferentiated carcinoma, unlike SS, is consisted with polygonal spindle cells and giant cells.[2] Although ectopic hamartomatous thymoma is composed of epithelial and spindle cells with adipose cells, the proportion of spindle cell is usually predominant.[11] Its spindle cells show naked nuclei, elongated ovoid nuclei with fine granular chromatin, and indistinct nucleoli or form nest, which are similar to SS. However, there are cytoplasmic processes in ectopic hamartomatous thymoma, unlike SS.[12] Cytologic features of fibroma and low-grade fibrosarcoma often reveal high cellularity with fibrocollagenous stroma in which tumor cells are embedded. Herringbone pattern clusters and individually scattered tumor cells are spindle-shaped and have scant cytoplasm. In intact specimen, the cytoplasmic processes of low-grade fibrosarcoma and fibroma are elongated at both ends of the cell.[1314] MPNST is composed of spindle cells, form long and short fascicles and in small clusters with some naked nuclei.[15] Diagnosis of FNA from MSS of thyroid gland might be very difficult because its cytologic features overlap with other spindle cell tumors mentioned above.
Cytogenetic and molecular genetic analysis is strong adjunctive method for the evaluation of soft tissue tumors, also important for confirming a diagnosis of SS. SS is characterized by a chromosomal translocation t(X; 18)(p11.2;q11.2), which results in the expression of a chimera SYT-SSX transcript.[8] Molecular genetic analysis is possible in formalin-fixed-paraffin embedded tissue as well as in FNA sample. Because FNA plays an important role in the diagnosis of thyroid lesions, it may be useful in evaluating thyroid spindle cell tumors.
SUMMARY
Primary MSS arising in the thyroid gland is extremely rare. Thus, diagnosis of MSS could be difficult with FNA even it is a useful tool in evaluating thyroid lesion. Clinical behavior of SS in thyroid gland is not clear although SS of soft tissue shows aggressive behavior. Therefore, early detection of MSS is important in determining treatment options including tumor resection and chemoradiation. When SS is suspected, detection of SYT-SSX fusion gene transcript is very helpful method for diagnosis.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Chang-Soo Park: Contributions to design; Eun-Hui Jeong: Acquisition of data; Young-Kim, Nah-Ihm Kim: Drafting the article or revising it critically for important intellectual content. Yoo-Duk Choi: Final approval of the version.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without identifiers, our institution does not require approval from Institutional Review Board.
LIST OF ABBREVIATIONS (In alphabetic order)
FNA - Fine needle aspiration
MPNST - Malignant peripheral nerve sheath tumor
MSS - Monophasic synovial sarcoma
SETTLE - Spindle epithelial tumor with thymus-like differentiation
SS - Synovial sarcoma.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model. (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Sharon W, Weiss JR, eds. Enzinger and Weiss's Soft Tissue Tumors (5th ed). Philadelphia, USA: Mosby; 2007. p. :1161-82.
- Synovial sarcoma of the thyroid. Report of a case with aspiration cytology findings and gene analysis. Acta Cytol. 2003;47:495-500.
- [Google Scholar]
- Primary synovial sarcoma of the thyroid gland: Case report and review of the literature. Case Rep Oncol. 2014;7:6-13.
- [Google Scholar]
- Fine needle aspiration cytology of thyroid swellings: How useful and accurate is it? Indian J Cancer. 2010;47:437-42.
- [Google Scholar]
- Fine-needle aspiration of synovial sarcoma: Criteria for diagnosis: Retrospective reexamination of 37 cases, including ancillary diagnostics. A Scandinavian Sarcoma Group study. Diagn Cytopathol. 2003;28:232-8.
- [Google Scholar]
- Monophasic synovial sarcoma: A cytologic spectrum. Diagn Cytopathol. 2004;30:19-23.
- [Google Scholar]
- Fine needle aspiration cytology of a spindle epithelial tumour with thymus-like differentiation (SETTLE) occurring in the thyroid. Cytopathology. 2012;23:413-5.
- [Google Scholar]
- Ectopic hamartomatous thymoma – A truly rare neoplasm: Report of a case. Surg Today. 2010;40:146-9.
- [Google Scholar]
- Fine needle aspiration cytology of ectopic hamartomatous thymoma. Acta Cytol. 2007;51:672-5.
- [Google Scholar]
- Mary K, Sidawy SZ, eds. Fine Needle Aspiration Cyology (1st ed). Philadelphia: Churchill Livingstone; 2007. p. :101-2.
- Fine-needle aspiration cytology of malignant peripheral nerve sheath tumors. Diagn Cytopathol. 2004;31:1-4.
- [Google Scholar]








