Translate this page into:
Fine needle aspiration of spindle cell ductal carcinoma in situ of the breast: A case report and the use of ancillary tests for the differential diagnosis of metaplastic carcinoma
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Spindle cell ductal carcinoma in situ (DCIS) is a recently recognized subtype of DCIS, which is associated with a very rare and unique morphology. Although the histologic features have been relatively well described in a few reports, the cytologic features have not. Even though the distinction of this lesion from usual DCIS is not crucial clinically, it should be noted that this lesion might simulate the features of metaplastic carcinoma on fine needle aspiration cytology. Here, we report a case of spindle cell DCIS in a 45-year-old female, with the detailed cytologic features, both on conventional and liquid-based preparations, along with some useful immunohistochemical staining markers for the differential diagnosis.
Keywords
Breast cancer
cytologic technique
cytology
fine-needle aspiration biopsy
noninfiltrating intraductal carcinoma
INTRODUCTION
The spindle cell variant of ductal carcinoma in situ (DCIS) of the breast is a recently recognized subtype of DCIS, which is characterized by an exclusive or predominant spindled appearance.[12] Although most morphologic variants of DCIS, such as solid, cribriform, comedo, and papillary DCIS, are relatively expectable findings considering the normal differentiation of the ductal epithelium of the breast, spindling, whorling, and fascicular arrangement of DCIS cells is a very unique and unusual finding.[3] In 2001, Farshid et al. first paid attention to this type of DCIS and described the histologic features in 17 cases.[1] Another study of 11 cases followed and reported that spindle cell DCIS often expresses varying degrees of certain neuroendocrine markers such as neuron-specific enolase (NSE), chromogranin, and synaptophysin.[2] In terms of the cytologic findings, however, to the best of our knowledge, only one case report has been reported to date.[4] The distinction of spindle cell DCIS from other, more common subtypes is clinically not crucial because their clinical behavior and prognosis do not significantly differ. However, caution is needed when we encounter this lesion in the cytologic samples in daily practice, as it may mimic a higher-grade lesion such as metaplastic carcinoma and may hence lead to unnecessary, more invasive treatment.[4]
Recently, we experienced such a case with striking spindle cell features in a 45-year-old female. Here, we report on this case, in which ancillary immunocytochemical staining using liquid-based preparation (LBP) was successfully applied, along with a review of the related literature.
CASE REPORT
A 45-year-old female presented with a palpable mass at the 7 o’clock position of the right breast. She had no significant medical or family history. The mass had been initially detected by ultrasonography as part of a routine health checkup 3 years earlier and was considered a benign lesion such as intraductal papilloma based on the ultrasonographic findings. At the follow-up visit to our department, the size of the mass had increased compared to that of the last examination, and the shape of the mass was more irregular and lobulated, with focal, ill-defined borders, suggesting the possibility of malignant transformation [Figure 1a]. Subsequently, fine needle aspiration (FNA) and ultrasonography-guided core needle biopsy of the lesion were performed. The lesion was diagnosed as low-grade DCIS, and preoperative positron emission tomography/computed tomography revealed focal fluorodeoxyglucose uptake at the same spot [Figure 1b]. The patient underwent breast-conserving surgery for the mass 1 week later.
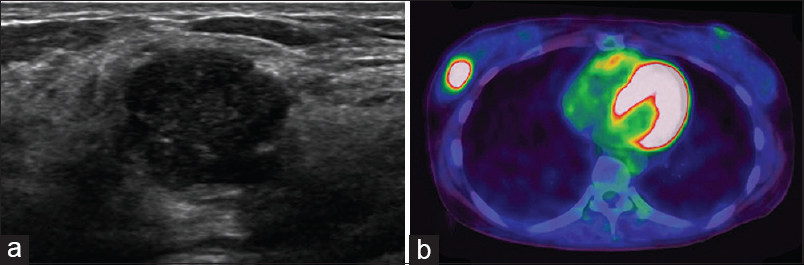
- Ultrasonographic and radiographic findings. (a) A relatively well-demarcated, lobulated, hypoechoic mass with mild heterogeneous echogenicity is noted, showing a focal, ill-defined border, suggesting the possibility of malignancy. (b) Positron emission tomography-computed tomography revealing focal fluorodeoxyglucose uptake in the same lesion
Both the conventional and LBP methods (SurePath, BD Diagnostics-TriPath Imaging, Burlington, NC, USA) were applied for the FNA material. Papanicolaou staining and hematoxylin and eosin staining were performed for the conventional smear, and Papanicolaou staining was used for the LBP slide.
The conventional smear was highly cellular and consisted of many irregular, “chunky” clusters of cohesive epithelioid-to-spindle cells and a few singly dispersed cells [Figure 2a and b]. The tumor cell clusters showed a whorling, fascicular, and streaming arrangement. The clusters showed relatively tight cohesion in the center, with peripheral feathery edges. Both the cells in the clusters and in the background showed medium-to-long spindle cytoplasm with oval and slightly angulated-to-slender, pencil-like, long nuclei. Predominantly, the cells showed variably enlarged hyperchromatic angulated nuclei with a high nuclear-to-cytoplasmic ratio [Figure 2b]. In some areas, the tumor cells showed polygonal or epithelioid shape and slightly angulated nuclei with one or two small nucleoli and occasional intranuclear pseudoinclusions [Figure 2c]. There was no evident background of tumor diathesis or singly dispersed bare nuclei of benign type throughout the slides. In the LBP slides, the tumor cells were tightly aggregated in three-dimensional clusters of metachromatically stained cells with more prominent hyperchromatic nuclei, suggesting a benign or malignant fibroepithelial tumor such as fibroadenoma or phyllodes tumor [Figure 2d]. In a few clusters, several tiny pseudoglandular structures of similar sizes, reminiscent of the cribriform pattern of DCIS, were observed [Figure 2e]. Mitosis was not identified throughout the smears. Based on these features, spindle cell DCIS, fibroepithelial tumor, myoepithelial tumor, and metaplastic carcinoma (spindle cell carcinoma) were suspected, and the diagnosis of low-grade DCIS of spindle cell type was made based on the histologic features of collaterally performed core needle biopsy.
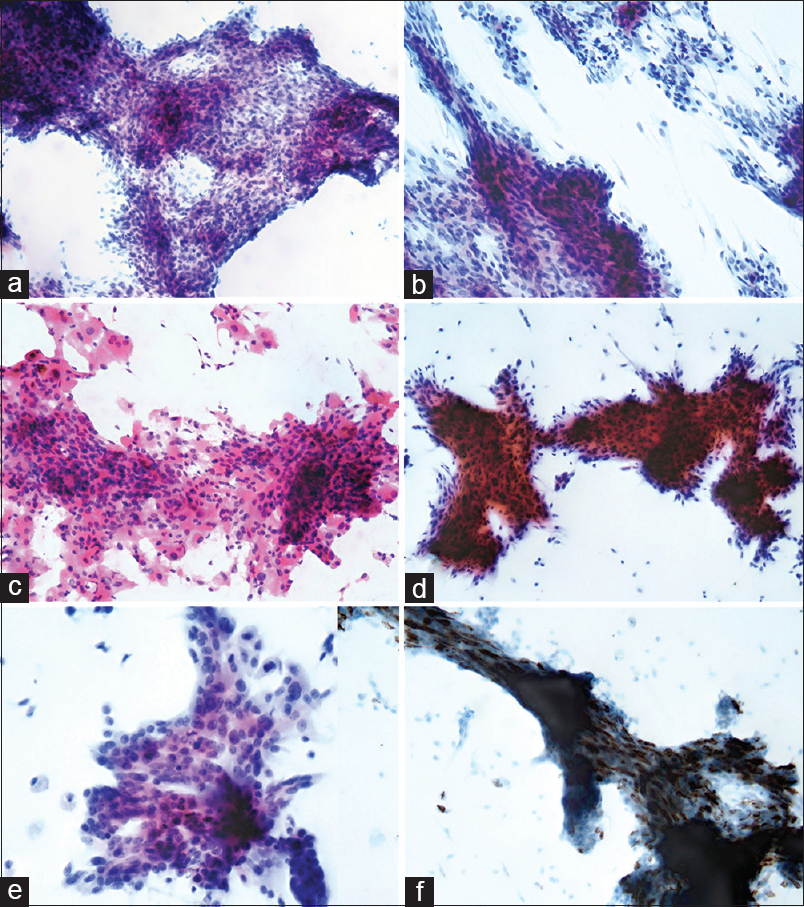
- Cytologic findings. (a) Conventional smear reveals many chunky clusters of hyperchromatic spindle cells with fascicular arrangement (Pap, ×200). (b) The spindle cells have angulated nuclei (×400). (c) Some polygonal-to-epithelioid cells are irregularly aggregated (H and E, ×400). (d) Liquid-based preparation reveals an apparent feathery appearance in the periphery with hyperchromatic atypical nuclei (Pap, ×200). (e) Some epithelioid cells forming pseudoglandular structure, reminiscent of cribriform ductal carcinoma in situ are noted (Pap, ×1K). (f) Immunohistochemical staining for CD56 using the liquid-based preparation shows strong positivity (×1K)
After reviewing the literature, immunocytochemical staining for neuroendocrine markers, CD56, NSE, synaptophysin, and chromogranin was performed using the LBP. As a result, scattered tumor cells in most clusters showed a strong positivity for CD56, whereas they were negative for the other markers [Figure 2f]. The immunocytochemical staining for smooth muscle actin was negative in most tumor cells except for in a few scattered myoepithelial cells. Moreover, the immunocytochemical staining for cytokeratin 5/6 was also negative, rejecting the possibility of myoepithelial tumors.
Histologic evaluation was performed on the resected breast. Grossly, the mass was a relatively well-demarcated, multinodular, white-yellow solid mass [Figure 3a]. It was surrounded by a thin fibrous tissue and showed a focal irregular border. Microscopically, it was an intraductal mass consisting of proliferation of ductal epithelium with striking spindle features [Figure 3b–d]. In <5% of the total tumor area, a focal area of cribriform DCIS was also found [Figure 3e]. Immunohistochemical staining for CD56 was positive, similar to in the cytologic samples, whereas the other neuroendocrine markers were negative. Immunohistochemical staining for p63 revealed diminished numbers of peripheral myoepithelial cells [Figure 3f]. Immunohistochemical staining for estrogen and progesterone receptor was positive, whereas c-erbB2 was negative. The Ki-67 labeling index was 3%.
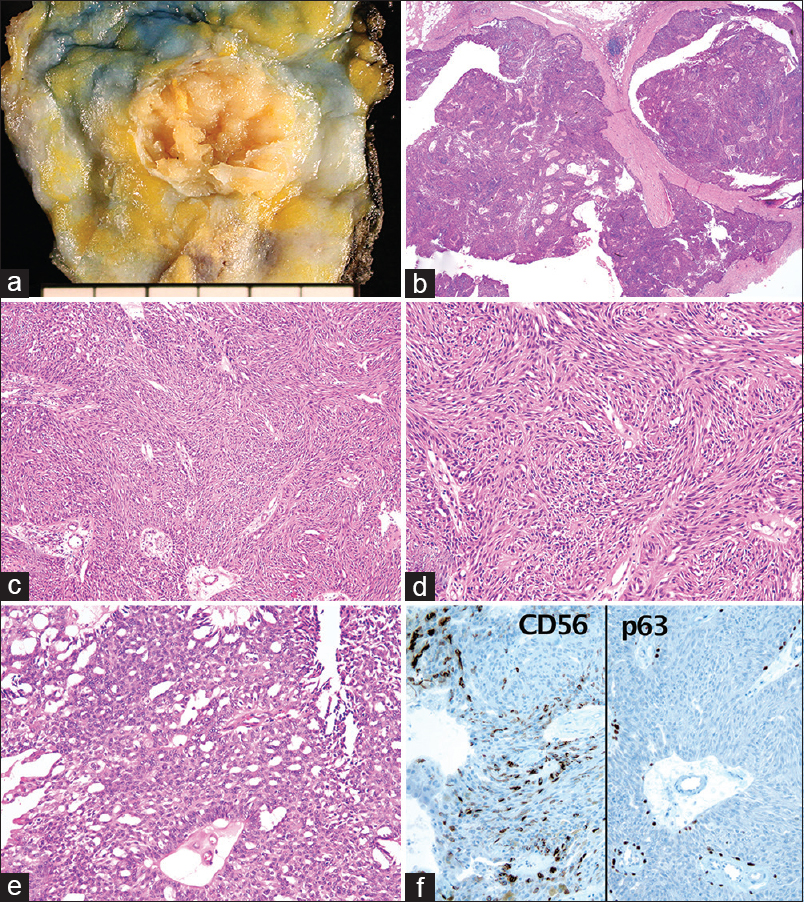
- Gross and microscopic findings. (a) A well-demarcated, multinodular, yellow-white solid mass surrounded by a thin fibrous capsule. (b) Microscopically, it is an intraductal hypercellular mass (H and E, ×15). (c) Monotonous proliferation of ductal cells showing a striking spindle appearance (×100). (d) Mostly, the tumor cells show a conspicuous whorling, fascicular, and streaming arrangement (×400). (e) Focal area shows an apparent cribriform pattern (×400). (f) Immunohistochemical staining for CD56 is positive and that for p63 reveals partial loss of myoepithelial cells (×400)
DISCUSSION
Spindle cell DCIS is very unique and interesting category of DCIS, not only because it is rare and was barely recognized until recently but also because the histologic features are quite unfamiliar and unexpected compared to those of the other histologic subtypes of DCIS.[13] Thus, if someone is not aware of the histologic features of this type, it can easily be misinterpreted as intraductal hyperplasia or a myoepithelial lesion.[12] Undoubtedly, appropriate recognition and accurate diagnosis of this subtype in FNA specimens is quite challenging, considering that the general specificity and sensitivity of FNA in the breast are not as good as those of core needle biopsy.[5]
Cytologically, spindle cell DCIS can mimic the features of both myoepithelial lesions and fibroepithelial tumors, a wide range of benign and malignant tumors that show biphasic or bipolar cells including fibroadenoma, benign and malignant phyllodes tumors, pleomorphic adenoma, adenomyoepithelioma, myoepithelial carcinoma, and metaplastic (spindle cell) carcinoma.[4] Especially, in our case, the absence of tumor diathesis because of the absence of necrosis in the lesion and the striking spindled appearance with extensive feathery edges were enough to give an impression of a fibroepithelial tumor. However, the cytologic atypia was more severe than that of fibroadenoma, and focal, loosely cohesive epithelioid cells with an occasional pseudoglandular (cribriform) pattern suggested DCIS rather than a usual fibroepithelial tumor. Furthermore, the absence of fragments of the haphazardly arranged, plump stromal cells is another important finding to exclude fibroepithelial tumors. For metaplastic carcinoma, most importantly, the cytologic atypia of the tumor cells was not too severe, and the absence of multinucleated giant cells and metachromatic amorphous materials were important clues for its exclusion. Myoepithelial lesions such as pleomorphic adenoma, adenomyoepithelioma, and myoepithelial carcinoma can be excluded by the absence of distinct biphasic cell populations, which consist of epithelial cells forming glands and long spindle cells with characteristic chondroid or hyalinizing collagenous stroma. However, it may be difficult, or even impossible, to make a definite diagnosis of spindle cell DCIS based on these cytologic findings alone, and in this case, we made the final diagnosis based on the histologic findings of the collaterally performed core needle biopsy.
Immunocytochemical demonstration of CD56, a neuroendocrine marker, in the LBP cytology is a novel finding of this case report. Although the spindle cell feature of DCIS was first reported by Farshid et al. in 2001, Tsang and Chan in 1996, described a subset of DCIS cases showing spindle cell features, while they moreover reported on endocrine DCIS, which shows neuroendocrine features immunohistochemically.[6] In their study, half of the cases showed spindle features in various portions. Farshid et al. also described the neuroendocrine immunophenotype in 8 among the total 17 cases in their study and found at least focal positivity, either for chromogranin or synaptophysin, in all 8 cases.[1] Similarly, a subsequent report by Tan et al. also showed various portions of positivity in 8 out of 11 cases, either for synaptophysin or for chromogranin.[2] In our case, scattered tumor cells in the clusters showed strong cytoplasmic positivity for CD56 but not for synaptophysin or chromogranin. However, in both the above-mentioned previous studies, the immunohistochemical phenotype of CD56 in spindle cell DCIS was not accessed. Considering that tumors with neuroendocrine features express various degrees of neuroendocrine markers immunohistochemically, the negative findings of synaptophysin and chromogranin in our case were fairly comprehensible compared to that in the previous research. Why spindle cell DCIS often expresses these neuroendocrine markers and how the spindle cell morphology relates to the neuroendocrine features in this tumor remain to be answered. The prognostic significance of neuroendocrine differentiation in breast cancers has not been yet clarified.[7] Nevertheless, it may be helpful to use immunocytochemistry of such neuroendocrine and myoepithelial markers in LBP cytology to support the proper diagnosis of spindle cell DCIS and to exclude myoepithelial lesions if one clearly is aware of this disease entity and its immunohistochemical characteristics.
SUMMARY
While the cytologic features of spindle cell DCIS are unique, they highly resemble those of myoepithelial and fibroepithelial lesions. Although the cytologic findings alone may not be enough for confirmative diagnosis of this lesion type, diagnosis of DCIS may be feasible upon proper exclusion of the possibility of other differential diagnoses, potentially using immunocytochemistry on LBP. The clinical significance of spindle cell DCIS and its relationship with endocrine DCIS should be further investigated.
COMPETING INTERESTS’ STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. WCP performed the fine needle aspiration biopsy and carried out the operation and the pre- and post-operative patient care. YC, YSL, TJK, CSK, and EJL were involved in the pathologic interpretation of the cytologic and surgical samples and confirmed the diagnosis. YC prepared the figures and drafted the manuscript. EJL critically revised the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This report was approved by the Institutional Review Board of The Catholic University of Korea, College of Medicine, Seoul, Republic of Korea (SC15ZISE0059).
LIST OF ABBREVIATIONS (In alphabetic order)
DCIS - Ductal carcinoma in situ
FNA - Fine needle aspiration
LBP - Liquid-based preparation
NSE - Neuron-specific enolase.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENTS
I appreciate Ms. Soon-Jin Ji for reviewing the manuscript style. This study was partly supported by the research grant from Institute of Clinical Medicine Research in the Catholic University of Korea, Yeouido St. Mary's Hospital.
REFERENCES
- Spindle cell ductal carcinoma in situ. An unusual variant of ductal intra-epithelial neoplasia that simulates ductal hyperplasia or a myoepithelial proliferation. Virchows Arch. 2001;439:70-7.
- [Google Scholar]
- Ductal carcinoma in situ with spindle cells: A potential diagnostic pitfall in the evaluation of breast lesions. Histopathology. 2004;45:343-51.
- [Google Scholar]
- International Agency for Research on Cancer, World Health Organization. WHO Classification of Tumours of the Breast. (4th ed). Lyon: International Agency for Research on Cancer; 2012.
- [Google Scholar]
- Cytologic findings of spindle cell ductal carcinoma in situ of the breast: A case report. Acta Cytol. 2005;49:323-6.
- [Google Scholar]
- Liquid-based cytology in fine-needle aspirates of the thyroid and breast. Korean J Pathol. 2009;43:99-106.
- [Google Scholar]
- Endocrine ductal carcinoma in situ (E-DCIS) of the breast: A form of low-grade DCIS with distinctive clinicopathologic and biologic characteristics. Am J Surg Pathol. 1996;20:921-43.
- [Google Scholar]
- Neuroendocrine differentiation and prognosis in breast adenocarcinoma. Histopathology. 2002;40:215-22.
- [Google Scholar]








