Translate this page into:
Cytological features of adenocarcinoma admixed with small cell neuroendocrine carcinoma of the uterine cervix
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Adenocarcinoma admixed with neuroendocrine carcinoma of the uterine cervix is a rare malignancy with a poor prognosis, and few reports have described the cytological features of this carcinoma. To characterize the cytological features of this malignancy in cervical smears, we report a case of a 52-year-old Japanese woman with cervical adenocarcinoma admixed with small cell neuroendocrine carcinoma (SCNEC). Cytologically, there were two types of cells with different sizes. The smaller cells formed clusters, which showed a partially Indian file pattern, a high nuclear/cytoplasmic ratio, and hyperchromatic nuclei. In contrast, the larger cells showed cytological features of adenocarcinoma, indicating a glandular-like pattern. Histological examination of biopsy specimens revealed that the tumors were composed of almost equal areas of SCNEC and adenocarcinoma. Neuroendocrine differentiation was confirmed by immunohistochemistry for synaptophysin and CD56. Thus, when adenocarcinoma cells are detected in smears, attempts to search for SCNEC cells should be made by combined cytological and histological analyses in order to reach an accurate diagnosis of the carcinoma in the uterine cervix.
Keywords
Adenocarcinoma admixed neuroendocrine differentiation
mixed carcinoma
neuroendocrine carcinoma
uterine cervix
INTRODUCTION
Cervical neuroendocrine carcinomas (NECs) are categorized into two groups, i.e., low-grade neuroendocrine tumors such as typical carcinoid and atypical carcinoid and high-grade NECs such as small cell NEC (SCNEC) and large cell NEC.[1] SCNEC comprises approximately 2% of all cervical cancers and is highly aggressive with early nodal and distant metastases, often resulting in a poor prognosis.[23] Most SCNECs present in a pure form, and only 4% of tumors are associated with adenocarcinoma.[45] Cytological studies of SCNEC have been reported in eight cases to date; of these eight cases, one showed as SCNEC with adenocarcinoma in the uterine cervix.[6] Even if the mixed-type is mainly composed of neuroendocrine-differentiated cells, it is defined as adenocarcinoma admixed with SCNEC by the World Health Organization (WHO) criteria. This is further complicated by the coexistence of other carcinomas, such as squamous cell carcinoma in situ (SCIS), which may exhibit a cytological morphology similar to that of SCNEC.[7] Here, we describe an extremely rare case of admixed carcinoma in the uterine cervix, including the cytological, histological, and immunohistochemical features.
CASE REPORT
A 52-year-old Japanese woman visited our hospital complaining of abnormal genital bleeding. Laboratory tests revealed elevated tumor markers as follows: carcinoembryonic antigen, 25.1 ng/mL (reference up to 5.0); CA125, 48 U/mL (reference up to 35); and squamous cell carcinoma (SCC) antigen, 1.8 ng/mL (reference up to 2.0). Magnetic resonance imaging revealed a cervical mass (8.0 cm × 7.2 cm × 4.9 cm) and a cystic ovarian mass. Malignant cells were detected through scraping cytology and resected biopsy from the endocervical area. Cytological evaluation led to a diagnosis of suspected NEC of the uterine cervix. However, biopsy yielded a diagnosis of adenocarcinoma admixed with NEC.
Smear cytology of the uterine cervix revealed distinct atypical cells in a proteinaceous debris-filled background with no streaming artifacts [Figure 1a]. The tumor cells were composed of two distinctly sized cells. The majority of the tumor cells were found to be of relatively smaller size than adenocarcinoma cells; these smaller cells presented predominantly as hyperchromatic crowded clusters with numerous single cells [Figure 1b]. Most clusters were loose and occasionally showed a partial molding arrangement. The most abundant cells, those with a high nuclear/cytoplasmic (N/C) ratio or lacking cytoplasm, were generally 1–2 times larger than the size of neutrophils. Nuclei of solid cell clusters were oval, thin-edged, often delicate, and generally small to moderate or slightly large in size. Analysis of the chromatin pattern of the nuclei indicated a mixture of euchromatin and coarsely granular chromatin with inconspicuous nucleoli.
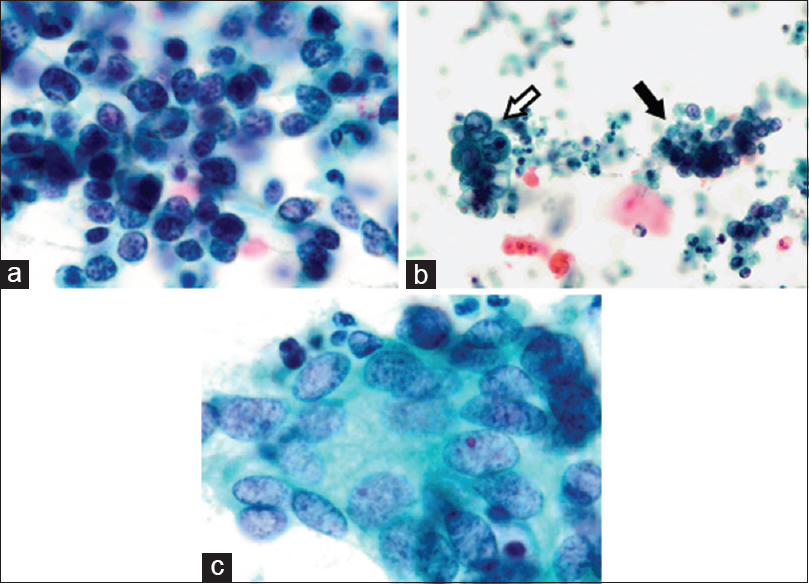
- Cytological findings from the uterine cervical smear. (a) Cluster of small cells with high nuclear/cytoplasmic ratios or lacking cytoplasm was observed. Nuclei of small cell neuroendocrine carcinoma show hyperchromatic, finely stippled nuclear features and an Indian file pattern. (b) Both adenocarcinoma (white arrow) and small cell neuroendocrine carcinoma (black arrow) cells were observed in the proteinaceous debris-filled background (c) Adenocarcinoma showing a gland-like pattern. Tumor cells showed abundant cytoplasm, unevenly distributed large nuclei, and ground glass chromatin (Pap, a and c: ×1000; b: ×400)
In contrast, tumor cells possessing unevenly distributed larger nuclei were observed admixed with SCNEC cells and were arranged in a tight, glandular-like pattern [Figure 1c]. The nuclei were 2–4 times larger than those of neutrophils and round to oval in shape. Their chromatin was granular, and the nuclei exhibited a moderate degree of anisokaryosis. Some of the nuclei contained prominent nucleoli. No abnormal keratinization was observed in any of the specimens. On the basis of these cytological findings, NEC was suspected.
Histologically, the tumor was comprised two adjacent components of almost equal area, containing either small-sized or moderately sized cells [Figure 2a]. The smaller cells exhibited a scant cytoplasm, and their nuclei were either round or elliptical with nuclear molding. Tumor cells exhibited nuclear hyperchromasia and inconspicuous nucleoli. In contrast, the area with moderately sized cells was found to be composed of solid, papillary, and tubular patterns accompanied by amorphous material and necrotic debris. These tumor cells harbored a thick cytoplasm, and the nuclei showed karyomegaly and were oval to elongated in shape, with prominent nucleoli. Immunohistochemically, areas showing a tubular pattern were positive for cytokeratin (CK) 19 [Figure 2b]. All neuroendocrine markers and MUC6 were negative in areas showing a tubular pattern (data not shown). We performed immunohistochemical staining of three neuroendocrine markers: CD56 (1B6, 1:800; Leica Biosystems, UK), synaptophysin (DAK-SYNAP, 1:400; Dako, CA, USA), and chromogranin-A (DAK-A3, 1:800; Dako); two squamous markers: CK14 (LL002, 1:320; Leica Biosystems) and p40 (polyclonal, 1:12,000; Calbiochem, Germany); two glandular markers: CK19 (RCK109, 1:400; Dako) and MUC6 (CLH5, 1:800; Leica Biosystems); and a human papillomavirus surrogate marker: p16 (G175-405, 1:800; BD Biosciences PharMingen, CA, USA). We found that the specimen was strongly positive for synaptophysin and CD56 in areas harboring smaller cells [Figure 2c and d], with no observable staining for chromogranin A (data not shown).
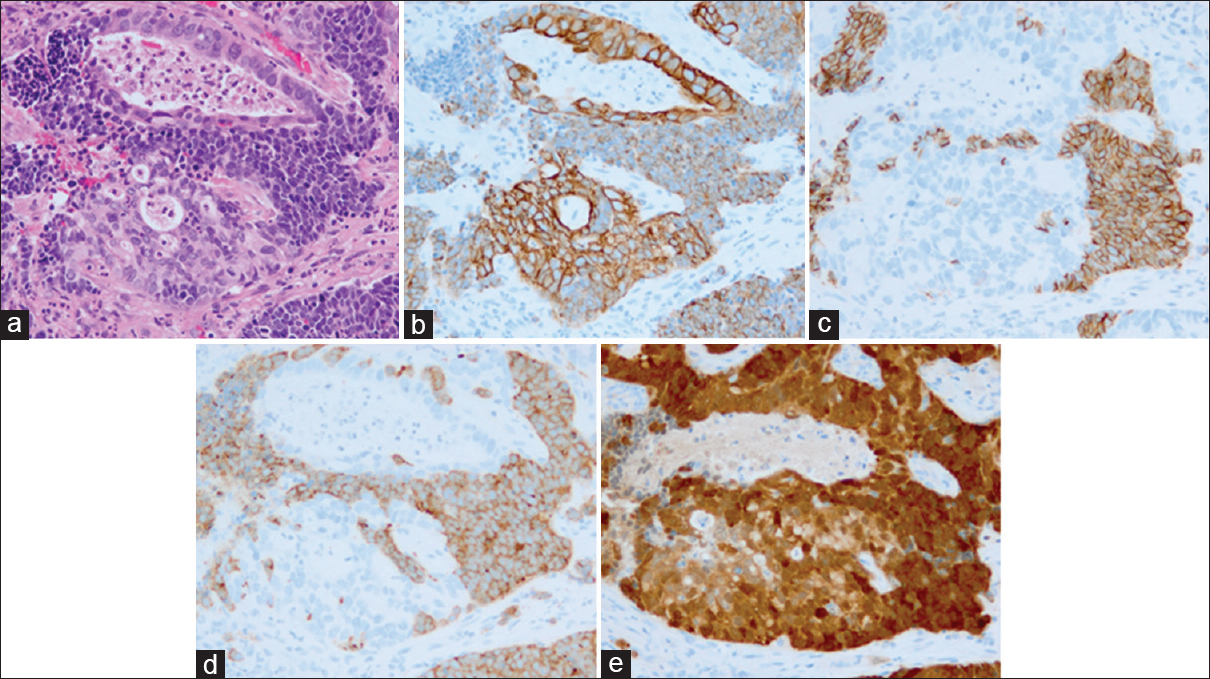
- Histologic findings of uterine cervical carcinoma. (a) Small cell neuroendocrine carcinoma was present in some adenocarcinomatous components, showing a tubular pattern (H and E). Surrounding tubular structures and individual tumor cells were small and showed a high nuclear/cytoplasmic ratio and round or elliptical nuclei with nuclear molding. (b-e) Immunohistochemistry demonstrating the expression of various molecular markers. (b) Cervical adenocarcinoma exhibiting diffuse cytoplasmic staining for cytokeratin 19. (c and d) Small cell neuroendocrine carcinoma exhibiting diffuse cytoplasmic staining for CD56 and synaptophysin, respectively. (e) Both small cell neuroendocrine carcinoma and adenocarcinoma exhibiting diffuse, strongly cytoplasmic, and nuclear staining with p16 (a-e, ×200)
In both tumor areas, mitotic figures were frequently observed, and almost all tumor cells were intensely stained immunohistochemically with p16 [Figure 2e]. No tendency toward keratinization was observed, and none of the cells expressed p40 and CK14 (data not shown).
DISCUSSION
In this report, we present a rare case of adenocarcinoma admixed with SCNEC. According to the current WHO classification, tumors showing neuroendocrine differentiation in association with variants of cervical adenocarcinoma are defined as “adenocarcinoma admixed with NEC,” in which the prognosis is similar to that of cervical SCNEC.[8] The cytological findings of this rare malignancy have been reported only a few times[6] because sampling through brush tool is not adequate for detecting SCNEC cells, which tend to show a subepithelial cell growth pattern.[9] In our case, “suspected NEC” was diagnosed by smear cytology because the vast majority of tumor cells were SCNEC-derived cells. Following cytological analysis, we reached a histological diagnosis and confirmed the presence of malignant glandular cells.
During cytological examination, SCNEC may be mistakenly diagnosed as the other malignancies, such as SCIS because of the rare occurrence of such tumors.[7] The cytological findings of SCIS include tumor cells with high N/C ratios, dense cytoplasm displaying keratinization, and scattered koilocytotic atypia and dysplasia against a clean background. In addition, in SCIS, the nuclei are hyperchromatic with coarsely granular and irregularly distributed chromatin. No nuclear molding is usually found in SCIS. Especially nuclear molding should be considered, made on the basis of the typical morphological features of SCNEC.
In addition to cytological screening, HPV typing techniques may also useful in the evaluation of patients who were diagnosed with cervical cancer. Serrano et al. demonstrated that HPV 18 and 45 are more frequently associated with adenocarcinoma and NEC than SCC.[10] The combination of cytological screening and HPV genotyping test may contribute to improving the diagnostic accuracy.
SUMMARY
We reported the cytological features of an extremely rare case of adenocarcinoma admixed SCNEC in the uterine cervix. We propose that the coexistence of SCNEC cells should be suspected when excess necrosis and smaller cells with high N/C ratios are detected with adenocarcinoma cells in the absence of koilocytotic atypia and dysplastic cells. Accurate diagnosis is critical because the therapeutic strategies for treating various tumors can differ greatly.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author of this manuscript declares that we qualify for authorship as defined by the ICMJE. Each author participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
Since this is a case report without identifiers, our institution does not require approval from the Institutional Review Board.
LIST OF ABBREVIATIONS (In alphabetic order)
HPV – Human papillomavirus
NEC – Neuroendocrine carcinoma
SCC – Squamous cell carcinoma
SCNEC – Small cell neuroendocrine carcinoma
SCIS – Squamous cell carcinoma in situ
WHO – World Health Organization.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENT
We gratefully acknowledge Masako Ichishi (Department of Pathologic Oncology, Mie University Graduate School of Medicine) for her skillful assistance in preparing immunohistochemical specimens. We also thank Alberto A. Gayle (Center for Medical and Nursing Education, Mie University) for his assistance in the revision of our manuscript.
REFERENCES
- IRAC WHO Classification of Tumours of Female Reproductive Organs 2014
- Small cell neuroendocrine carcinoma of the cervix: Outcome and patterns of recurrence. Gynecol Oncol. 2004;93:27-33.
- [Google Scholar]
- Neuroendocrine tumors of the genital tract. Am J Clin Pathol. 2001;115(Suppl(1)):S94-112.
- [Google Scholar]
- Small-cell neuroendocrine carcinoma of the uterine cervix associated with micro-invasive squamous cell carcinoma and adenocarcinoma in situ. Pathol Int. 1996;46:520-5.
- [Google Scholar]
- Mixed endocervical adenocarcinoma and high-grade neuroendocrine carcinoma of the cervix with ovarian metastasis of the former component: A report of 2 cases. Int J Gynecol Pathol. 2012;31:490-6.
- [Google Scholar]
- Neuroendocrine small cell carcinoma of the cervix associated with endocervical adenocarcinoma: A case report. Acta Cytol. 2007;51:589-93.
- [Google Scholar]
- Differential diagnostic features of small cell carcinoma in the uterine cervix. Diagn Cytopathol. 2008;36:618-23.
- [Google Scholar]
- Adenocarcinoma of the uterine cervix: Why is it different? Curr Oncol Rep. 2014;16:416.
- [Google Scholar]
- Carcinoid of the uterine cervix: Additional observations on a new tumor entity. Cancer. 1976;38:2328-42.
- [Google Scholar]
- Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012;7:38.
- [Google Scholar]








