Translate this page into:
Diagnosis of sebaceous lymphadenoma by fine needle aspiration in a patient with Cowden syndrome: Case report and review of the literature
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Sebaceous lymphadenoma (SLA) is a rare benign tumor of the salivary gland that commonly arises in the parotid gland in adults. It is rarely diagnosed correctly preoperatively. In addition, to the best of our knowledge, SLA has not been described yet in the literature in association with Cowden's syndrome (CS). We present an extremely rare case of parotid SLA that was diagnosed preoperatively by fine needle aspiration in a patient with CS.
Keywords
Fine needle aspiration
lymphoadenoma
parotid
salivary glands
sebaceous
INTRODUCTION
Sebaceous lymphadenoma (SLA) of the salivary glands is a rare benign tumor that is most commonly found in the parotid gland. Histologically, it is characterized by islands of epithelial cells showing sebaceous differentiation in a lymphoid background. More commonly, fine needle aspiration (FNA) diagnosis preoperatively of SLA is nonspecific, and these have been interpreted as a variety of benign conditions in the literature.[1] To best of our knowledge SLA, has not been reported in a patient with Cowden's syndrome (CS). We report a case of SLA diagnosed correctly by FNA in a 57-year-old male with CS.
CASE REPORT
A 57-year-old male presented with left parotid mass. The patient noticed the mass 3 weeks before the presentation. The mass was painless without overlying redness of the skin, fever or any drainage from the area. The patient had a past medical history significant for Lhermitte Duclos disease (dysplastic gangliocytoma of the cerebellum), arteriovenous malformation of vertebral artery in the cervical spine region, chronic sinus disease, hypothyroidism, hyperplastic gastric polyps, hamartomatous colonic polyps, kidney stones, benign kidney cysts, hyperuricemia, hyperlipidemia, hypertension, and prostatic adenocarcinoma. Hamartam atous lesions diagnosed in this patient are consistent with the clinical syndrome of CS.
The patient was referred to radiologist for ultrasound guided biopsy. The ultrasound showed a heterogeneous, solid mass predominantly 1.9 cm × 1.6 cm × 1.4 cm with areas of isoechogenicity and hypoechogenicity within the superficial superior aspect of the left parotid gland. The mass appeared to be well circumscribed with minimal vascularity and no appreciable internal vascularity. In addition, regional ipsilateral cervical adenopathy along deep jugular vein was noted. A follow-up magnetic resonance imaging of the neck revealed a heterogeneous T1- and T2-weighted signal with an enlarged intraparotid lymph node worrisome for a neoplasm.
Fine needle aspiration was performed with ultrasound guidance and immediate adequacy triage. Three air dried Diff Quik, and three alcohol fixed Papanicolau stained smears were done. The aspirate smears showed moderate cellularity and were composed of epithelial cells clusters with microvesicular cytoplasm and small wrinkled nuclei identifiable as sebocytes. There were also foci of squamous metaplasia admixed with nonneoplastic salivary gland tissue in the background of abundant lymphocytes. There was no cytologic evidence of malignancy. The sebaceous cells differentiation was more evident in the air dried, Diff Quik smears. Intraprocedural differential diagnoses included sebaceous lymphadenoma, sebaceous adenoma and in addition other benign salivary tumors with sebaceous differentiation to be ruled out. The alcohol fixed Papanicoloau smears showed clusters of sebocytes (described above) admixed with lymphocytes in the background [Figures 1–4]. Foci of squamous metaplasia was identified. Flow cytometry was attempted on the specimen but proved to be inadequate.
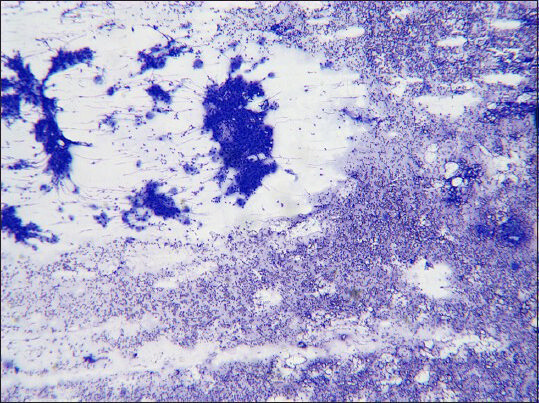
- Epithelial clusters admixed with lymphocytes in the background (Diff Quik, ×4)
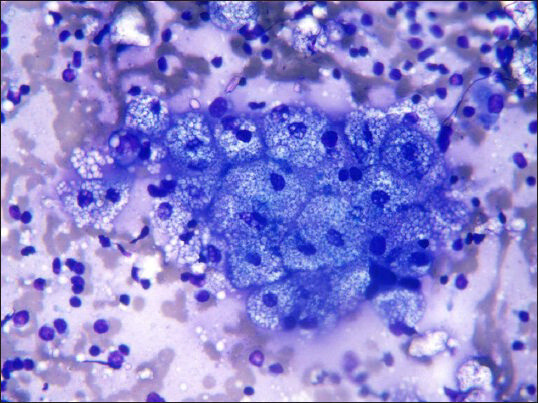
- Epithelial cell clusters with abundant cytoplasm and small nuclei; numerous lymphocytes are present in the background (Pap, ×20)
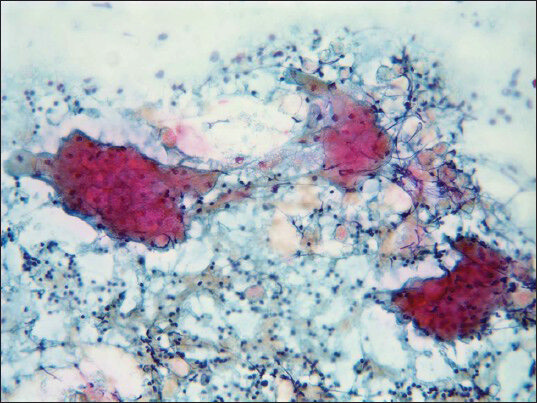
- Microvesicular cytoplasm: Characteristic of sebocytes (Diff Quik, ×40)
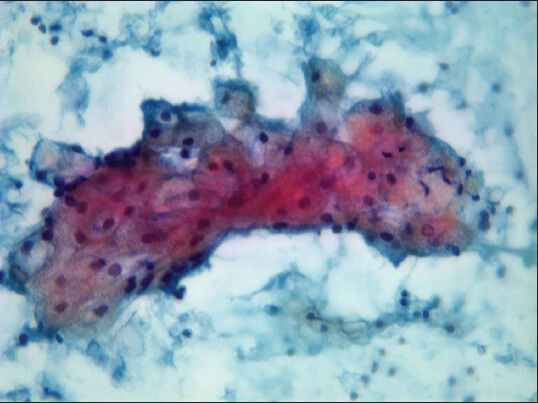
- Microvesicular cytoplasm: Characteristic of sebocytes (Pap stain, ×40)
The patient underwent left superficial parotidectomy with preservation of the facial nerve. Gross examination of the resected specimen revealed a yellow variegated well circumscribed mass measuring 2 cm × 1.9 cm × 1.5 cm. No discreet lymph node was noted in the submitted specimen.
Microscopically the tumor was well defined and mostly encapsulated with an epithelial cystic component and a lymphoid component. The epithelial component was composed of squamous and sebaceous differentiation with no significant atypia or evidence of malignancy. The lymphoid component was dense and showed reactive lymphoid follicles with germinal centers [Figures 5–6]. The parotid tissue outside the tumor area was unremarkable. Previous fine needle biopsy site was identified by granulation tissue and multinucleated giant cells within the mass.
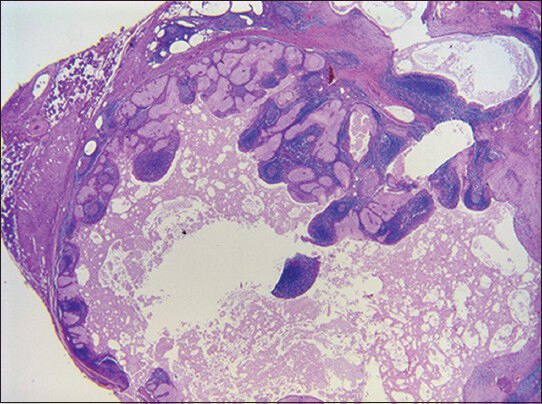
- Sebaceous lymphadenoma (H and E, ×10)
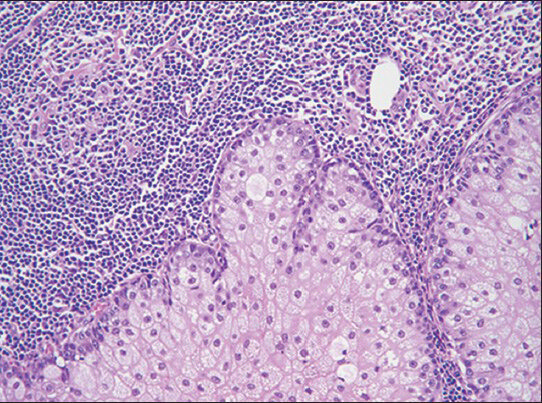
- Sebaceous lymphadenoma (H and E; ×20)
The final diagnosis was sebaceous lymphadenoma. There was no history of recurrence at 20 months follow-up.
DISCUSSION
In our case, three distinct components were identified in the aspirate; epithelial cells with microvesicular cytoplasm, squamous epithelium suggestive of metaplastic origin and a prominent lymphoid infiltrate. Lack of nuclear atypia favored consideration of a benign neoplasm. No other epithelial or mesenchymal differentiation was noted.
The first step in the differential diagnosis was to determine whether the FNA findings represented a salivary gland neoplasm with a lymphoid component, or this represented an intraparotid lymph node lesion sampling. Intraprodecural communication with the interventional radiologist confirmed the former impression. Next based on the composite of three different cell types, differential diagnosis included a neoplasm with sebaceous and lymphoid component, for example, sebaceous lymphadenoma, a primary sebaceous epithelial neoplasm, for example, sebaceous adenoma with inadvertent sampling of adjacent lymph node, or a benign salivary gland neoplasm with sebaceous component lacking other differentiation in the submitted sample, for example, pleomorphic adenoma with sebaceous differentiation. Lack of nuclear atypia supported the clinical and imaging impression of a benign neoplasm. There are six reported cases of sebaceous lymphadenocarcinoma.[2] The FNA interpretation was available in one of them and did not help as it suspected a benign lesion, and the subsequent histologic evaluation revealed a typical SLA with transition to a sebaceous adenocarcinoma.[3]
It is very important to differentiate SLA from low grade mucoepidermoid carcinoma (MEC) which is the most common primary malignant malignancy of the parotid gland. In the FNA smears, there is a mixture of cell types in MEC including glandular, squamous and intermediate. Unlike SLA, it would be unusual to have dominant lymphoid background in MEC, and usually the pools of mucin may surround the glandular cells, while the mucin in SLA if present will be limited to the ductal cells and within the ductal lumens.[4] Mucin stain would be helpful in this regard and Oil red O will be positive in the sebaceous cells. Foreign body giant cell reaction is more commonly seen in SLA.
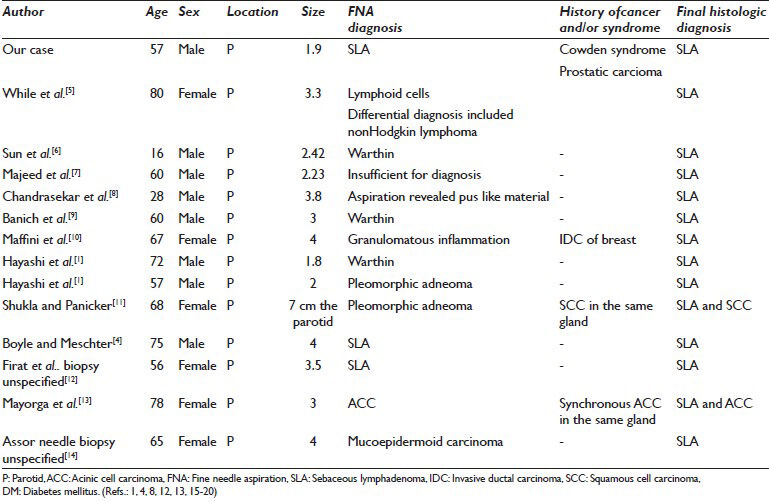
To our knowledge, this is the second case in the literature where a preoperative diagnosis has been made on an FNA specimen [Table 1].
Sebaceous lymphadenoma is a rare benign neoplasm of the salivary gland with about <50 cases reported in the English literature.[15] It was first described by Rawson and Horn in 1950 and was coined with its name in 1959 by Mcgavran.
The origin of SLA is still controversial; possible sources being branchial cleft remnants, sebaceous metaplasia of the epithelial component of Warthin's tumor, or ectopic salivary gland inclusions in a lymph node.[1016] The latter theory is based on the fact that the presence of subcapsular marginal sinuses and germinal centers within the lymphoid background in SLA, as in Warthin's tumor, makes it more likely to be derived from ectopic salivary gland tissue in a lymph node.[16]
Sebaceous lymphadenoma has no sex predilection, and the majority of the cases were discovered after the age of 50 years with only two cases reported in the pediatric population.[615] About 90% of the cases were described in the parotid gland or periparotid lymph nodes with rare cases reported in the minor salivary glands of the lip, maxilla, and anterior neck.[10171819] Usually it presents as a progressively enlarging, painless mass lasting from <1 month up to 15 years or as an incidental finding.[10] SLA is rarely recurrent, and complete surgical excision is the treatment of choice.[6] Only one case recurred after unspecified initial excision technique.[12]
Fine needle aspiration has been shown to be both sensitive (ranging from 85.5% to 99%) and specific (ranging from 96.3% to 100%) in identifying parotid tumors but that certainly depends on the experience of the cytologist. The importance of preoperative diagnosis in such cases lies in the fact that it can be helpful in assessing the need for subsequent surgery and the likely extent of the surgery.[9]
As mentioned before, SLA was rarely diagnosed preoperatively [Table 1]. This may be due to the synchronous occurrence adjacent to another lesion or the rare nature of the disease leading to under diagnosis of this entity.[4] To our knowledge, in addition to our case, two previous reported cases had correct preoperative diagnosis. One of these was diagnosed by FNA and the other one by unspecified type of biopsy.[412] Similar to our case, the FNA findings of the former one reported by Boyle and Meschter[4] revealed a mixed population of lymphoid cells including plasma cells and tangible body macrophages with scattered three-dimensional, cohesive aggregates of epithelial cells, many demonstrating the characteristic cytoplasmic vacuolization of sebocytes, surrounded by layer of basaloid cells without mitosis or cellular pleomorphism.
Another clinical aspect of interest in this case was the preexisting diagnosis of CS in this patient.
Cowden syndrome is a rare inherited autosomal dominant condition with 80% of patients having a germ-line mutation of the phosphatase and tensin homologue tumor suppressor gene. CS is characterized by multiple hamartomas in a variety of tissues from all three embryonic layers. It is a cancer predisposition syndrome with an increased risk of developing malignancy in many tissues especially breast, thyroid, and endometrium. It was first described in 1963 by Lloyd and Dennis in a report of a patient called Rachel Cowden. One of its pathognomonic criteria is the presence of adult Lhermitte Duclos disease which was seen in our patient.[20]
To the best of our knowledge, we are the first to report SLA in a patient with a CS.
CONCLUSION
Sebaceous lymphadenoma is a rare benign tumor of the salivary gland that can be diagnosed accurately in adequately sample FNA specimen. Furthermore, SLA should be included in the differential diagnosis when evaluating smears from salivary gland tumors with a sebaceous component. This would be very useful in directing optimal further management.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
All the authors declare that they have no competing interests
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article. Each author acknowledges that this final version was read and approved
ETHICS STATEMENT BY ALL AUTHORS
As this is case report without identifiers, our institution does not require approval from Institutional Review Board (IRB) (or its equivalent).
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Sebaceous lymphadenoma of the parotid gland: Report of two cases and review of the literature. Acta Otorhinolaryngol Ital. 2007;27:144-6.
- [Google Scholar]
- A red cheek as first clinical sign of a sebaceous lymphadenocarcinoma of the parotid gland with lymphangiosis carcinomatosa and lymph node metastases. Am J Dermatopathol. 2011;33:e50-3.
- [Google Scholar]
- Sebaceous lymphadenocarcinoma of parotid gland. Eur Arch Otorhinolaryngol. 2006;263:940-2.
- [Google Scholar]
- Fine needle aspiration cytology of a sebaceous lymphadenoma: A case report. Acta Cytol. 2004;48:551-4.
- [Google Scholar]
- Sebaceous lymphadenoma: A case report and review of the literature. Ear Nose Throat J. 2010;89:E22-3.
- [Google Scholar]
- Sebaceous lymphadenoma of parotid gland in a child. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:253-5.
- [Google Scholar]
- Sebaceous lymphadenoma of the parotid gland. Dentomaxillofac Radiol. 2008;37:300-4.
- [Google Scholar]
- Unilocular cystic sebaceous lymphadenoma: A rare tumour. Ann R Coll Surg Engl. 2007;89:1-3.
- [Google Scholar]
- Sebaceous lymphadenoma identified by fine needle aspiration biopsy: A case report. Acta Cytol. 2007;51:211-3.
- [Google Scholar]
- Sebaceous lymphadenoma of salivary gland: A case report and a review of the literature. Acta Otorhinolaryngol Ital. 2007;27:147-50.
- [Google Scholar]
- Synchronous sebaceous lymphadenoma with squamous cell carcinoma-Case report. World J Surg Oncol. 2003;1:30.
- [Google Scholar]
- Synchronous ipsilateral sebaceous lymphadenoma and acinic cell adenocarcinoma of the parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:593-6.
- [Google Scholar]
- Sebaceous lymphadenoma of the parotid gland. A case report. Am J Clin Pathol. 1970;53:100-3.
- [Google Scholar]
- Sebaceous lymphadenoma of the parotid gland in a 13-year-old girl: A case report. Head Neck Pathol. 2010;4:144-7.
- [Google Scholar]
- Sebaceous neoplasms of salivary gland origin. Report of 21 cases. Cancer. 1984;53:2155-70.
- [Google Scholar]
- Sebaceous lymphadenoma of minor salivary gland origin. A case report. Ala J Med Sci. 1973;10:453-4.
- [Google Scholar]
- Sebaceous lymphadenoma of the lip: Report of a case of minor salivary gland origin. J Oral Pathol Med. 2002;31:242-3.
- [Google Scholar]
- Sebaceous lymphadenoma in the midline of the maxilla: Report of case. J Oral Maxillofac Surg. 1999;57:1461-3.
- [Google Scholar]








