Translate this page into:
The cytopathologic features of mammary analog secretory carcinoma and its mimics
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Mammary Analogue Secretory Carcinoma (MASC) is a newly recognized neoplasm of the salivary gland, first described in 2010. This tumor harbors a unique translocation, t(12;15)(p13;q25) that results in the fusion of ETV6 with NTRK3 which produces a transformative chimeric tyrosine kinase. To date, few cases of MASC sampled by fine needle aspiration have been reported. Cytologically, MASC can be confused with other oncocytic salivary gland tumors, including Warthin-tumor, acinic cell carcinoma (AciCC) and mucoepidermoid carcinoma. It is characterized by a monomorphic population of lesional cells with round nuclei, prominent nucleoli and abundant, eosinophilic foamy cytoplasm; forming papillary groups with transgressing vessels. Though, based on cytomorphology alone, the definite diagnosis can be challenging, in conjunction with available clinical clues (i.e. male patient, extra-parotid site) MASC should be included in the differential diagnosis of FNA specimens diagnosed as oncocytic salivary gland neoplasms or suspicious for AciCC. Here we present a case of MASC with FNA sampling at our institution.
Keywords
Fine-needle aspiration
mammary analog secretory carcinoma
oncocytic salivary gland neoplasms
INTRODUCTION
Since 2010 when the seminal paper by Skálová et al. was published,[1] many authors have begun to catalog the diagnosis and clinicopathologic characteristics of mammary analogue secretory carcinoma (MASC). However, while the literature concerning the histological characteristics of MASC is burgeoning,[2345] the cytomorphological features of MASC and its addition to the differential diagnosis of a salivary gland fine-needle aspiration (FNA) specimen by cytopathologists is just emerging. Until date, few cases of MASC sampled by FNA have been reported;[678910] with one lesion diagnosed via cytological analysis (the remaining cases received a final diagnosis of MASC after histological evaluation).[7] Here we present a case of MASC with FNA sampling at our institution.
CASE REPORT
The case we present here is about a 51-year-old female who presented with an asymptomatic parotid mass found on routine examination. Computed tomography scan showed a hyper-attenuating 0.8 × 0.7 cm lesion in the superficial lobe of the right parotid gland, which was confirmed on magnetic resonance imaging. Ultrasound examination of the lesion demonstrated a solid, hypoechoic mass with minimal vascularity. The patient underwent FNA at an outside institution and a diagnosis of “consistent with pleomorphic adenoma (PA)” was rendered. The FNA slides were reviewed at our institution for surgical management (notably the Diff quick preparation from the outside institution was of poor quality). The FNA specimen showed a monomorphic population of lesional cells arranged in papillary groups with transgressing vessels [Figure 1]. The lesional cells demonstrated round nuclei, prominent nucleoli and foamy cytoplasm [Figure 2]. The case was re-classified as “salivary gland neoplasm, low grade” with differential diagnosis of acinic cell carcinoma (AciCC) and low grade mucoepidermoid carcinoma (MEC).

- Fine-needle aspiration cytology smear demonstrating a monomorphic population of cells with eosinophilic cytoplasm arranged in papillary groups (Papanicolaou, ×40)
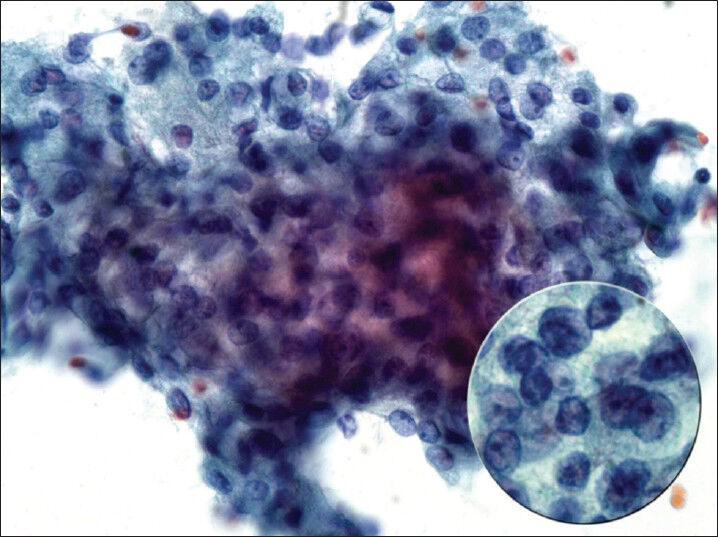
- Fine-needle aspiration cytology smear demonstrating lesional cells with round nuclei, prominent nucleoli and foamy cytoplasm (Papanicolaou, ×60, inset, ×100)
The patient subsequently underwent resection and the mass was received for histological evaluation. The lesion appeared as a well circumscribed gray-tan nodule within the superficial parotid gland, measuring 0.8 cm in greatest dimension. The histological examination displayed a circumscribed, unencapsulated lobulated mass with microcystic and tubular structures [Figure 3]. The microcystic structures contained bubbly, eosinophilic secretions. The tumor cells had pale eosinophilic granular cytoplasm and low grade nuclei with distinct nucleoli [Figure 4]. Mitotic figures were rare to absent. Focal invasion of the surrounding parotid gland was noted; however no perineural invasion or lymphovascular invasion was identified. A mucicarmine stain was negative, while periodic acid Schiff with and without diastase failed to demonstrate intracytoplasmic glycogen, and highlighted secretory material in microcystic spaces. As MASC was suspected, this material was tested for ETV6 rearrangement by fluorescence in situ hybridization (FISH), which demonstrated an atypical ETV6 rearrangement in 167/200 cells (83%), confirming the diagnosis.
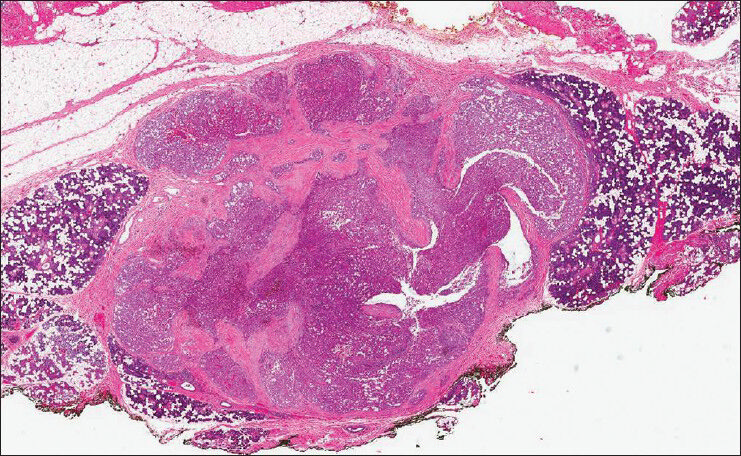
- Histologically, the tumor is a circumscribed, unencapsulated lobulated mass with microcystic and tubular structures (H and E, ×2.5)
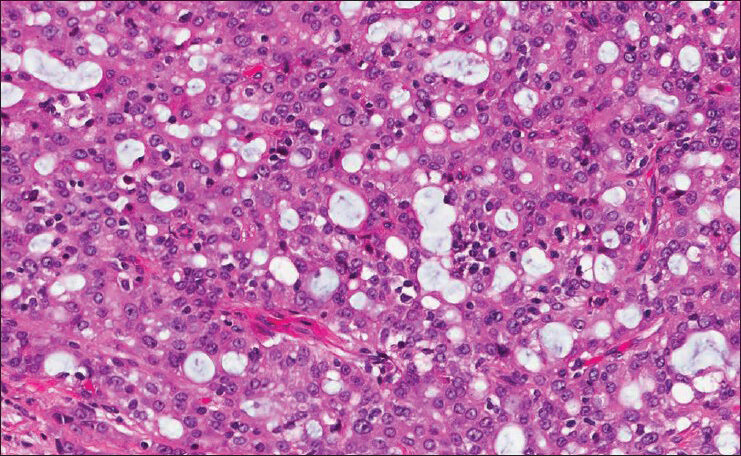
- Histologically, the microcystic structures contain bubbly, eosinophilic secretions; the tumor cells have pale eosinophilic granular cytoplasm and low grade nuclei with distinct nucleoli (H and E, ×40)
DISCUSSION
Mammary analogue secretory carcinoma, first described in 2010,[1] is a newly recognized neoplasm of the salivary gland. As its name implies, it shares morphologic and cytogenetic characteristics with its counterpart in the breast. Occurring most commonly in the parotid, MASC is a low grade neoplasm often confused with AciCC, particularly the “granular poor” variant. Unlike AciCC, MASC is strongly positive for S100 and mammoglobin and negative for DOG-1. MASC also differs from AciCC in its clinical presentation, occurring more commonly in males, frequently associated with lymph node metastases, and is thought to be more common in extra-parotid sites. This tumor harbors a unique translocation, t(12;15) (p13;q25) that results in the fusion of ETV6 with NTRK3, which produces a transformative chimeric tyrosine kinase. Although present in several pathologic processes, if identified, this translocation is thought to be diagnostic of MASC in the appropriate pathologic setting.
Cytologically, MASC is often confused with other oncocytic salivary gland tumors, including primarily AciCC and MEC, but also can rarely be misclassified as salivary duct carcinoma (SDC) and PA.[7] Similar to the cases in the current literature,[678910] our case demonstrated a monomorphic population of lesional cells forming papillary groups with transgressing vessels. The individual cells demonstrated round nuclei with prominent nucleoli and abundant, eosinophilic foamy cytoplasm. Overall, the appearance was that of a low grade tumor, as pleomorphism and mitotic figures were not noted.
While MASC and AciCC can have similar architectural patterns, MASC may show evidence of mucin production, a feature not seen in AciCC. Furthermore, according to some authors, MASC is more likely to demonstrate greater variation in the size of cytoplasmic vacuoles.[7] When compared to MEC, particularly the oncocytic variant, MASC can share features of mucin production and eosinophilia. However, architectural patterns such as papillae with transgressing vessels are more characteristic of MASC and cells tend to have multivacuolation, as opposed to the univacuolation reported in cases of MEC.[7]
Salivary duct carcinoma and PA can very rarely be confused with MASC. SDC often will exhibit nuclear pleomorphism and tumor diathesis, revealing its high grade nature and distinguishing it from MASC. In those rare cases that appear less clearly malignant, SDC will still often demonstrate greater size variation and decapitation secretions (cell block).[7]
Although on the other end of the neoplastic spectrum of SDC, PA can also rarely be confused with MASC (as occurred originally in the present case). The mucin production in MASC may be so abundant as to be confused with the myxoid stroma of PA. However, in most cases it should be clearly distinguishable from the metachromatic material seen in PA. The key, and often present, features include the papillary architecture and distinct granular cytoplasm seen in cases of MASC.
CONCLUSION
Though, based on cytomorphology alone, the definite diagnosis can be challenging, in conjunction with available clinical clues (i.e., male patient, extra-parotid site) MASC should be included in the differential diagnosis of FNA specimens diagnosed as oncocytic salivary gland neoplasms or suspicious for AciCC. This creates an opportunity for ancillary studies such as immunohistochemistry and/or FISH analysis on cell block material, which may lead to the appropriate classification of the lesion prior to surgical resection.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article. TDS, VAL and ZB were each directly involved in the diagnosis of this case. All authors read and approved the final manuscript. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
As this is case report without identifiers, our institution does not require approval from Institutional Review Board (IRB) (or its equivalent).
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model.(authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599-608.
- [Google Scholar]
- Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology. 2012;61:387-94.
- [Google Scholar]
- Searching for mammary analog secretory carcinoma of salivary gland among its mimics. Mod Pathol. 2014;27:30-7.
- [Google Scholar]
- Most nonparotid “acinic cell carcinomas” represent mammary analog secretory carcinomas. Am J Surg Pathol. 2013;37:1053-7.
- [Google Scholar]
- Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: Expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012;36:27-34.
- [Google Scholar]
- Cytological features of mammary analogue secretory carcinoma of salivary gland: Fine-needle aspiration of seven cases. Diagn Cytopathol 2014 epublication ahead of print, doi: 10.1002/dc. 23139
- [Google Scholar]
- The cytological features of mammary analogue secretory carcinoma: A series of 6 molecularly confirmed cases. Cancer Cytopathol. 2013;121:234-41.
- [Google Scholar]
- Aspiration biopsy of mammary analogue secretory carcinoma of accessory parotid gland: Another diagnostic dilemma in matrix-containing tumors of the salivary glands. Diagn Cytopathol. 2014;42:49-53.
- [Google Scholar]
- Mammary analogue secretory carcinoma: The first submandibular case reported including findings on fine needle aspiration cytology. Head Neck Pathol. 2012;6:135-9.
- [Google Scholar]
- Cytopathologic features of mammary analogue secretory carcinoma. Cancer Cytopathol. 2013;121:228-33.
- [Google Scholar]








