Translate this page into:
Endoscopic ultrasound and endobronchial ultrasound-guided fine-needle aspiration of deep-seated lymphadenopathy: Analysis of 1338 cases
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
We retrospectively studied 1338 samples of lymph nodes obtained by endoscopic and endobronchial ultrasound-guided fine needle aspiration biopsy (EUS and EBUS-FNAB) with an objective of characterizing the utility of this diagnostic modality in the assessment of deep-seated lymphadenopathy. The secondary aims were to establish the utility in the diagnosis of lymphoma and to determine the number of passes required to obtain adequate cellularity for flow cytometric analysis.
Materials and Methods:
On-site assessment was performed by a cytopathologist using Diff-Quik (American Scientific Products, McGraw Park, IL) stain. In addition, Papanicolaou and immunohistochemical stains were performed and additional samples were sent for flow cytometric analyses (n = 145). The final cytologic diagnosis was correlated with surgical pathology diagnosis and/or clinical follow-up. In select cases, fluorescence in situ hybridization analysis with specific probes was performed on Diff-Quik smears.
Results:
Both morphology as well as ancillary studies (flow cytometry or immunohistochemical stain and/or fluorescence in situ hybridization) show that EUS and EBUS-FNA are effective techniques to detect and stage intrathoracic and intra-abdominal tumors. Operating characteristics show that these are highly sensitive (89%) and specific (100%) techniques for the diagnosis of lymphoma. At least two passes provided an average of 5.66 million cells (range, 0.12-62.32 million) for lymphoma cases.
Conclusions:
EUS and EBUS-FNA are powerful modalities to stage malignancies and at least two passes can provide adequate cells for flow cytometric analysis. We also demonstrate that fluorescence in situ hybridization analysis can be performed on Diff-Quik-stained and mounted smears.
Keywords
Endoscopic ultrasound
endobronchial ulatrsound
fine-needle aspiration
mediastinum
flow cytometry
fluorescence in situ hybridization
lymph node
lymphoma
INTRODUCTION
Finding an accurate and cost-effective method for tissue acquisition and analysis of deep-seated lymphadenopathy remains a subject of continued investigation. In recent years, the introduction of endoscopic ultrasound-guided fine-needle aspiration (EUSFNA) as well as endobronchial ultrasound-guided fine-needle aspiration (EBUSFNA) has allowed for the sampling of deep tissues, including deep-seated lymph nodes.[1–4] These modalities have been demonstrated to be highly sensitive and specific tools in the diagnosis of metastatic neoplasms in deep-seated lymph nodes.[124–7]
Earlier studies have also shown that FNA biopsy (FNAB) combined with flow cytometry is a rapid and cost-effective alternative in the diagnosis of non-Hodgkin lymphoma (NHL) in palpable lymph nodes.[38–12] Although FNA of deep-seated lymphadenopathies has proven to be a powerful diagnostic tool, many investigators are still uncertain of its utility in the diagnosis of NHL. The most cited drawbacks include inadequate cellularity for the performance of ancillary studies and the loss of tissue architecture. Some have even suggested that FNA does not always provide reliable diagnoses and perhaps may even mislead therapy.[13] It is well accepted that the diagnosis of NHL relies upon a combination of immunomorphologic findings with a heavy reliance on immunohistochemical stains on formalin-fixed paraffin-embedded tissue or flow cytometry analyses on fresh specimens. Although studies suggest that FNA can obtain sufficient lymphoid cells to perform flow cytometric analyses, there is a paucity of data in the literature documenting the cellular yield of lymphoid cells obtained utilizing either EUS-FNA or EBUS-FNA for the performance of ancillary studies.
Flow cytometry requires tens to hundreds of thousands of cellular events to provide a diagnosis. The results of this technique can often provide quick and strong corroborative evidence of a specific NHL on cells obtained utilizing EUS-FNA. Fluorescence in situ hybridization (FISH) analysis performed on Diff-Quik-stained and mounted smears can provide additional valuable information, particularly when FNA is performed in the staging of a NHL with specific translocations.
In the current study, we document our experience in the assessment of EUS- and EBUS-guided FNA of deep-seated lymphadenopathies in a large group of samples. In addition, we also studied the utility of these modalities in the diagnosis of hematologic malignancies. Furthermore, we also sought to characterize the yield of lymphoid cells obtained using these modalities to perform flow cytometric and FISH analyses.
MATERIALS AND METHODS
A retrospective study was undertaken and a computer-generated search found three thousand six hundred and eighty four (3 684) EUS and EBUS-FNAB cases. All cases were performed with a curvilinear echoendoscope (UC-30P, Olympus, Mellville, NY) at our institution from January 2002 to June 2009. Of these, 1 338 (36%) were from deep-seated lymph nodes, 1 248 (34%) from the pancreas, and 1 098 (30%) from other sites including gastrointestinal tract, hepatobiliary tree, adrenal gland, kidney, spleen, and lung. For each sample, immediate cytologic evaluation (ICE) was performed by a cytopathologist utilizing Diff-Quik (American Scientific Products, McGraw Park, IL)-stained smears in the endoscopy suite to ensure specimen adequacy, provide onsite diagnosis, and suggest appropriate triage of the specimen when indicated. In addition, another cytologic smear prepared on site was fixed in alcohol and stained with Papanicolaou stain. Aspirated material was placed in 10 ml of Hanks balanced salt solution (Invitrogen, GIBCO, Grand Island, NY), and a cell block was prepared using the fibrin clot method, fixed in formalin, and embedded in paraffin. Additional immunohistochemical stains were performed when indicated on sections from the cell block. In cases where a lymphoproliferative process was suspected at the time of FNA, one additional pass was collected in 10 ml Hanks balanced salt solution for 4-color flow cytometry (Becton Dickinson FACSort, San Jose, CA). Final diagnoses were correlated with histological examination of surgical specimens and/or clinical follow-up. Flow cytometry data, including the number of passes and number of viable cells, were also evaluated on a subset of cases.
Furthermore, in select cases, FISH analysis was performed retrospectively on Diff-Quik-stained and mounted slides prepared from EUS-FNABs as a proof of principle to demonstrate whether it can be performed on such samples. First, the slides were demounted by soaking in xylene for 1 to 3 days to facilitate coverslip removal. Then, the slides were destained and the cells fixed by immersing them in a series of jars (5 minutes in each) containing (i) xylene, (ii) xylene-absolute ethanol, (iii) absolute ethanol, and (iv) methanol-acetic acid. The slides were then air-dried and examined under a phase-contrast microscope to identify an area with adequate cell density. Interphase FISH analysis was then performed using the Vysis LSI IGH/CCND1 dual-fusion probe set for the t(11;14)(q13;q32) and the Vysis LSI IGH/BCL2 dual-fusion probe set for the t(14;18)(q32;q21) (Abbott Molecular, Des Plaines, IL). Slide hybridization and washes were performed according to the manufacturer's instructions. The slides were then counterstained with DAPI and analyzed with an Olympus BX61 microscope (Olympus America, Inc., Melville, NY) equipped with the appropriate filter combination and a CCD camera, and coupled to the CytoVision image analysis system (Applied Imaging, Santa Clara, CA). Fifty to one hundred interphase nuclei were analyzed on each slide.
RESULTS
In keeping with our earlier reports,[214] no more than five passes (median of three passes) were performed for lymph node aspirates to obtain a diagnosis. A total of 1 338/3 684 (36%) EUS and EBUS-FNABs were obtained from deep-seated lymphadenopathies from intrathoracic (n = 918, 68.6%) and intra-abdominal (n = 420, 31.4%) sites [Table 1]. Of all the sites in the thoracic cavity, the most frequently aspirated lymph nodes were subcarinal (n = 568, 61.9%) and aortopulmonary window (n = 115, 12.5%) lymph nodes. Two of the most commonly aspirated sites in the abdomen were peripancreatic (n = 124, 29.5%) and celiac (n = 124, 29.5%) lymph nodes.
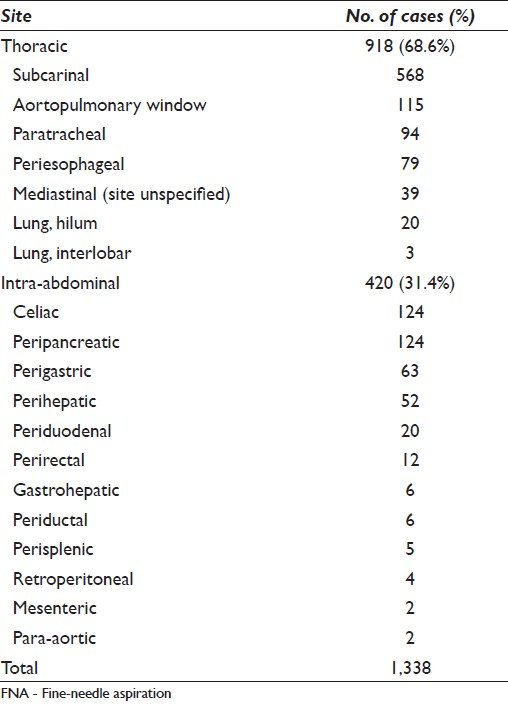
The majority of the 1 338 cases were rendered a diagnosis of negative for malignancy (n = 852, 63.7%) while 486 of the cases (36.3%) were diagnosed as positive for malignancy [Table 2]. Of all malignancies, 51 (10.5%) were given a diagnosis of hematopoietic malignancy, including NHL, either primary or recurrent.
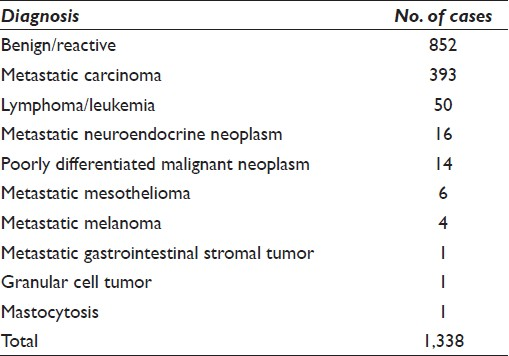
Samples were collected for flow cytometric analysis from 145 patients (10.8%) via EUS-FNAB at the time of ICE for either a clinical and/or morphologic suspicion of a hematopoietic malignancy. These cases had the following distribution on final analysis: benign lymph node (n = 81, 55.8%), lymphoma (n = 46, 31.7%), nodal metastases of non-hematologic malignancies (n = 17, 11.7%), and leukemia (n = 1, 0.7%).
Patient demographics of deep-seated lymphoma/leukemia cases
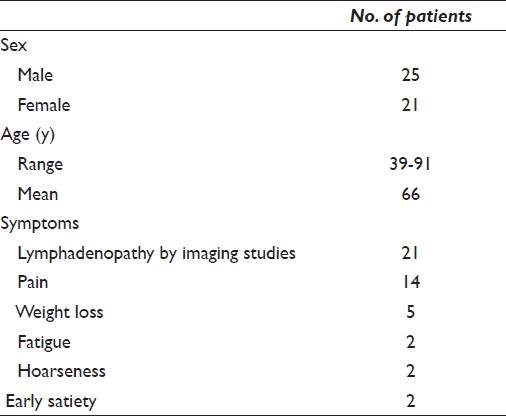
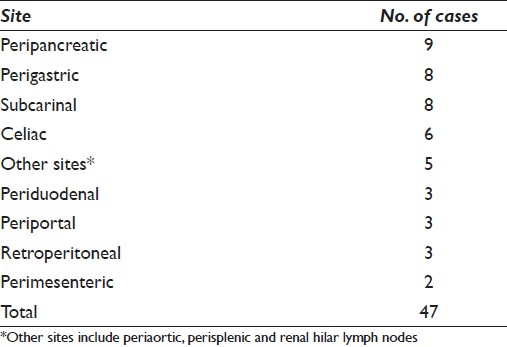
Fifty-one cases from 46 patients with deep-seated lymphoma/leukemia included those that represented primary diagnosis (n = 30, 65.2%) as well as recurrent lymphoma/leukemia (n = 16, 34.8%). This includes one case of recurrent hairy cell leukemia in a gastro-hepatic lymph node in a patient previously considered in remission.[15] Of the 46 patients with deep-seated lymphoma, 25 (54.3%) were men and 21 (45.7%) were women with an age range of 39 to 91 years (mean age, 66 years). The signs and symptoms reported in these patients included abdominal pain (11 patients), weight loss (5 patients), chest pain (3 patients), hoarseness (2 patients), early satiety (2 patients), and fatigue (2 patients; [Table 3]). In 21 (45.6%) of these patients, EUS-FNA was performed due to unsuspected lymphadenopathy that was detected on imaging studies. The locations of lymph nodes aspirated included various thoracic and intra-abdominal sites [Table 4].
Endoscopic ultrasound-guided fine-needle aspiration biopsy diagnosis of deep-seated lymphoma
Cytologic and flow cytometric diagnoses on the 46 cases of lymphoid neoplasms were compared with the gold standard diagnosis rendered on surgical pathology specimens. At the time of ICE, the majority of preliminary diagnoses rendered included monomorphous, polymorphous, or atypical lymphoid population. A diagnosis of suspicious for lymphoma was given in a subset of cases with significant atypia or monotony of the lymphoid cell population. Forty-three of 46 cases (93.5%) were diagnosed as NHL and three cases were diagnosed as Hodgkin lymphoma (6.5%). Of the 43 cases of NHL, 18 were B-cell lymphoma, not otherwise specified, eight were large B-cell lymphoma, seven were chronic lymphocytic leukemia/small lymphocytic lymphoma CLL/SLL; [Figure 1] 6 were follicular center cell lymphoma FCC; [Figure 2] 1 was mantle cell lymphoma, 1 was extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALToma) involving a lymph node; and 2 were T-cell lymphoma on FNA samples [Table 5].
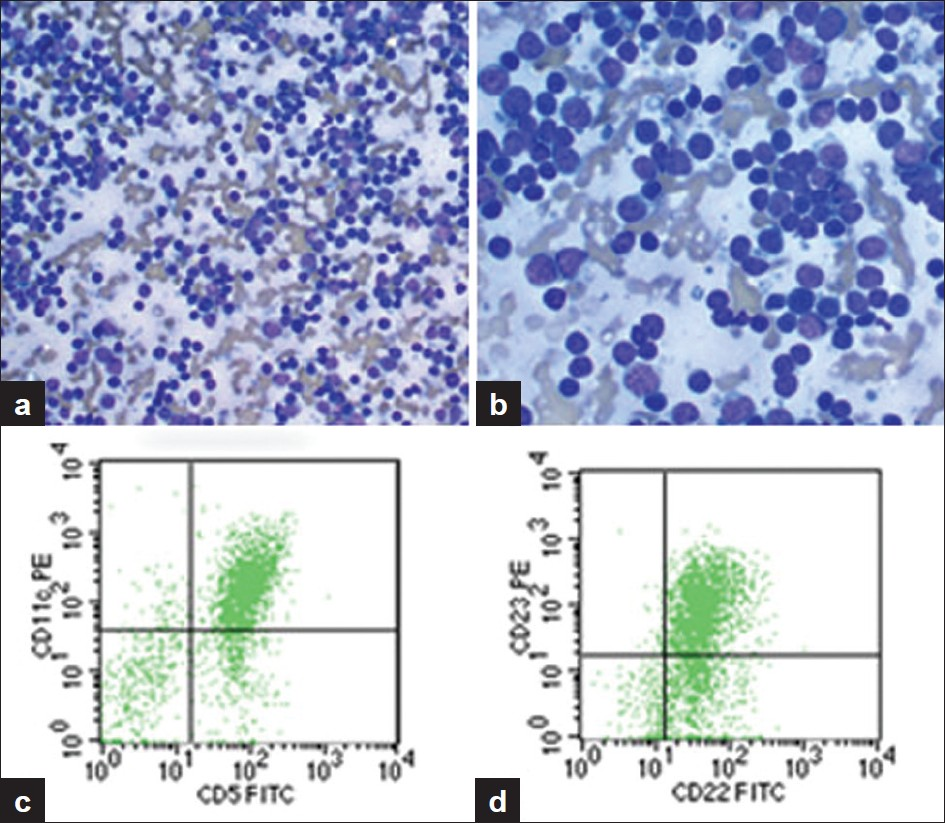
- A case of CLL/SLL. a) Aspirate smear from an enlarged subcarinal lymph node showing a moderately cellular smear with single small, relatively monomorphous cells (Diff-Quik, ȕ200), b) Aspirate smear showing small cells with clumped chromatin (Diff-Quik, ȕ400), c) Subcarinal lymph node specimen. Scattergram showing distribution of CD5 positivity and CD11c positivity in the gated population of cells, d) Scattergram showing distribution of CD22 positivity and CD23 positivity in the gated population of cells. FITC, fluorescein isothiocyanate; PE, phycoerythrin
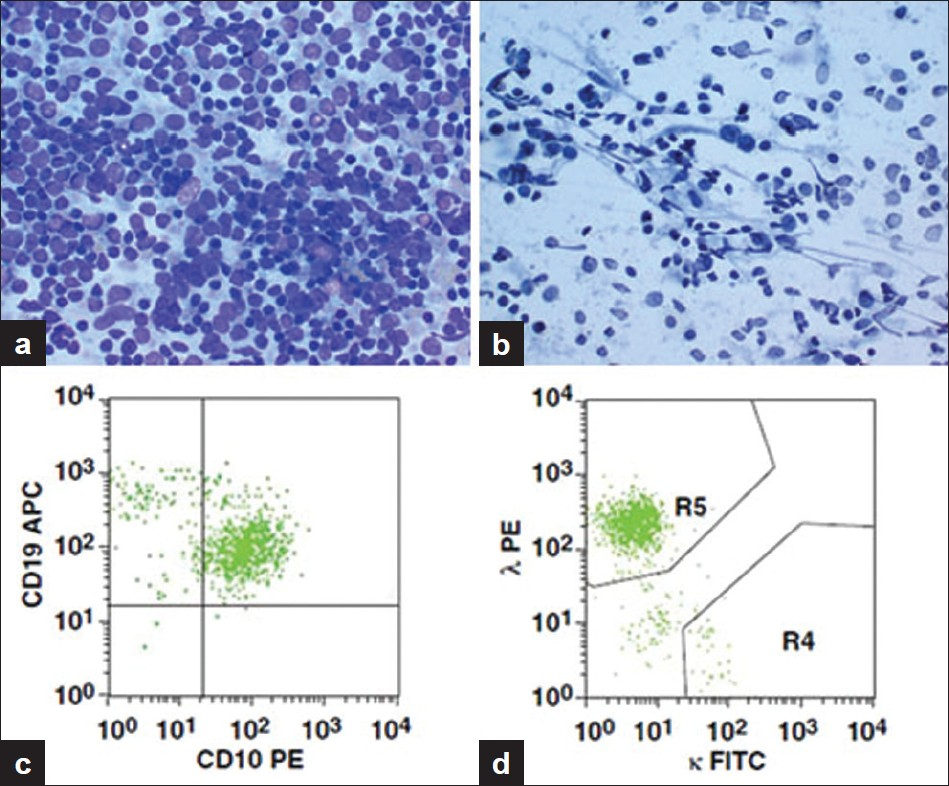
- A case of FCC. a) Aspirate smear from an enlarged peripancreatic lymph node showing a highly cellular smear with a polymorphous population of cells (Diff-Quik, ×400), b) Aspirate smear showing large and small cells, some with prominent nucleoli (Papanicolaou, ×400), c) Peripancreatic lymph node specimen. Scattergram showing distribution of CD10 positivity and CD19 positivity in the gated population of cells, d) Scattergram showing distribution of κ and λ light chain positivity with a predominance of λ light chain in the gated population of cells. APC, allophycocyanin
In 40 of 43 cases of NHL, flow cytometry was performed on the EUS-FNAB sample. The total yield of viable cells per case of NHL diagnosed by flow cytometry ranged from 0.12 to 62.32 million (mean, 5.66 million cells). The total yield of viable cells per case of low-grade lymphomas (B-cell lymphoma not otherwise specified, CLL/SLL, FCC, mantle cell, marginal zone, and T-cell lymphoma) ranged from 0.12 to 62.32 million (mean, 6.26 million cells), whereas the total yield of viable cells per case of high-grade lymphoma (large B-cell lymphoma) ranged from 0.17 to 1.66 million (mean 0.76 million cells; [Table 6]). Full panels were performed on 25 cases (62.5%) and limited panels were performed on 15 cases (37.5%). A diagnosis of NHL was rendered in 41 of 43 (95%) FNA samples by using cytomorphologic studies in combination with either flow cytometry or immunohistochemical stains (4 of 41 cases) performed on cell blocks. In one particular case of NHL diagnosed on FNA samples, the determination of a small but distinct monoclonal B-cell population by flow cytometry helped clinch the diagnosis. EUS-FNA yielded insufficient cellularity to perform flow cytometric analysis in three (6.5%) cases of lymphoma.
In one of these cases, we could not provide a definitive diagnosis of NHL on FNA. However, the patient had a history of weight loss and diffuse lymphadenopathy, with a previous EUS revealing a large mass in the greater curvature of the stomach. An EUS-FNAB was performed on a peripancreatic lymph node, and a lymphoproliferative process was noted cytomorphologically. However, only three passes could be obtained, and the aspirated sample sent for flow cytometry yielded a quantity of cells insufficient for analysis. The patient subsequently underwent surgical biopsy, and a high-grade B-cell lymphoma of the stomach was found.
In the second case, the patient presented with abdominal pain and CT scan demonstrated matted lymph nodes near the renal vein. EUS-FNAB of a periportal lymph node was performed, and a diagnosis of reactive lymph node was rendered. The aspirated sample submitted for flow cytometry yielded a quantity of cells insufficient for analysis. The patient then underwent surgical biopsy which yielded a diagnosis of FCC with diffuse large B-cell transformation.
Of all 46 cases positive for a lymphoid malignancy, three cases were diagnosed as Hodgkin lymphoma. The cytomorphology in combination with supportive immunohistochemical stains led to a diagnosis of recurrent Hodgkin lymphoma in one case. In this case, an atypical lymphoid population including large, atypical cells were present in the aspirate. CD15 and CD30 immunostains were performed on the cell block. Morphology, immunohistochemical stains and the clinical history of previous Hodgkin lymphoma led to the correct diagnosis. In the other two cases, a specific diagnosis of Hodgkin lymphoma could not be rendered by cytomorphology and were considered false-negative results since both EUS-FNAB cases were read as benign lymph nodes. In both cases, scant lymphoid material was present in the aspirate and large, atypical Reed-Sternberg cells were not identified. Subsequent surgical pathology samples provided the definitive diagnoses.
Our results show that endoscopic-guided FNA is a highly sensitive (89%) and specific (100%) modality with high positive predictive (100%) and negative predictive (94%) values [Table 7].

Endoscopic ultrasound-guided fine-needle aspiration biopsy diagnosis of non-lymphoid neoplastic lesions in lymph nodes
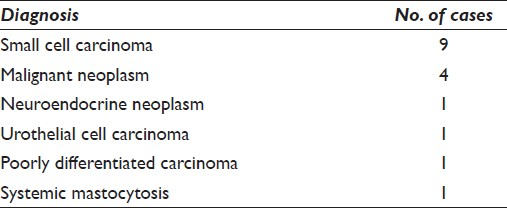
Seventeen of 145 cases of deep-seated lymphadenopathies were diagnosed as non-lymphoid neoplasms in lymph nodes. Nine (52.9%) were diagnosed as small cell carcinoma, four (23.5%) as positive for malignant neoplasm, one as neuroendocrine neoplasm, one as urothelial cell carcinoma, one as poorly differentiated carcinoma, and one as systemic mastocytosis.[16] The last case was reported by our group previously. Of the 17 patients with neoplastic lesions within lymph nodes, 11 were men and six were women with an age range of 36 to 79 years (mean age, 60 years; [Table 8]). In all 17 cases, flow cytometry was performed to rule out a possible lymphoma. By using cytomorphologic studies on aspirated samples in combination with immunohistochemical stains performed on cell blocks, all 17 cases of deep-seated neoplasms within lymph nodes were correctly diagnosed.
In 14 of the 17 cases, the total yield of viable cells for flow cytometry per case ranged from 0.02 million to 307.89 million (mean, 23.66 million cells). Full panels were performed on seven cases (50%) and limited panels were performed on seven cases (50%). The remaining three cases submitted for flow cytometry yielded a quantity of cells insufficient for diagnosis [Table 6].
In 15 of 17 cases, immunohistochemical studies were performed on cell blocks which aided in classification of the neoplastic lesions. Seven of the nine cases of small cell carcinoma were most consistent with pulmonary origin with positive staining for TTF-1. In the four cases diagnosed as malignant neoplasm by EUS-FNAB, one was further classified as metastatic hepatocellular carcinoma by surgical biopsy and another was classified as large cell neuroendocrine carcinoma by surgical biopsy.
Patient demographics of reactive lymph node cases
Of the 81 benign lymph node aspirates from 78 patients, 31 were diagnosed as reactive lymphoid hyperplasia and 50 as benign. They were obtained from 45 men and 33 women with an age range of 4 to 88 years (mean age, 55.2 years). FNA was performed in these cases for unexplained deep-seated lymphadenopathy.
In these cases, the total yield of viable cells per case for flow cytometry ranged from 0.003 million to 212.85 million (mean, 5.85 million cells). Full panels were performed on 43 of the cases (60.6%) and limited panels were performed on 28 of the cases (39.4%). Ten of 81 cases submitted for flow cytometry yielded a quantity of cells insufficient for diagnosis. In the 70 cases with sufficient cellularity for flow cytometry, no clonal population was detected [Table 6]. Six of 81 cases demonstrated granulomatous inflammation. These cases stained negative for fungal and acid fast organisms.
One of these showed an aberrant B-cell population by flow cytometric analysis. This population of cells expressed CD45, CD19, CD20, CD22, and CD23, but not surface or cytoplasmic immunoglobulin or terminal deoxynucleotidyl transferase. These findings could represent a reactive process, a lymphoproliferative process, or progressive transformation of a germinal center.
Fluorescence in situ hybridization analysis of endoscopic ultrasound-guided fine-needle aspiration biopsy of lymphoma cases
FISH analysis was performed retrospectively on Diff-Quik-stained and mounted slides on seven cases (four cases of FCC and three cases of mantle cell lymphoma). FISH analysis using the IGH/BCL2 dual-fusion probe set for the t(14;18)(q32;q21) was performed on the FCC lymphoma cases. Our results demonstrated that of the four FCC lymphoma cases, FISH analysis for IGH/BCL2 gene fusion was positive for one case [Figure 3a], negative for one case, and was unsuccessful in the remaining two cases. FISH analysis using the IGH/CCND1 dual-fusion probe set for the t(11;14)(q13;q32) was performed on the mantle cell lymphoma cases. Of the three mantle cell lymphoma cases, FISH analysis demonstrated IGH gene rearrangement but without IGH/CCND1 gene fusion for one case suggesting the possible fusion of the IGH locus with another gene [Figure 3b]. FISH analysis was unsuccessful in the other two cases. The unsuccessful cases failed to demonstrate any hybridization signals, in spite of the presence of DAPI-stained nuclei, which suggests that the DNA was most likely degraded. Interestingly, two of the three cases that yielded interpretable FISH results were obtained by EUS-FNA less than one year prior to the FISH test performance. All four cases where FISH analysis yielded unsuccessful results, the specimens were obtained by EUS-FNA more than two years before the FISH test was performed. The most likely explanation for these failures is DNA degradation, which is most likely due to the combined effect of old age and mounting/demounting of the slides with prolonged exposure to xylene in the demounting process to facilitate coverslip removal. This suggests that should one consider performing FISH analysis on Diff-Quik-stained and mounted slides, then it should be undertaken as early as possible.
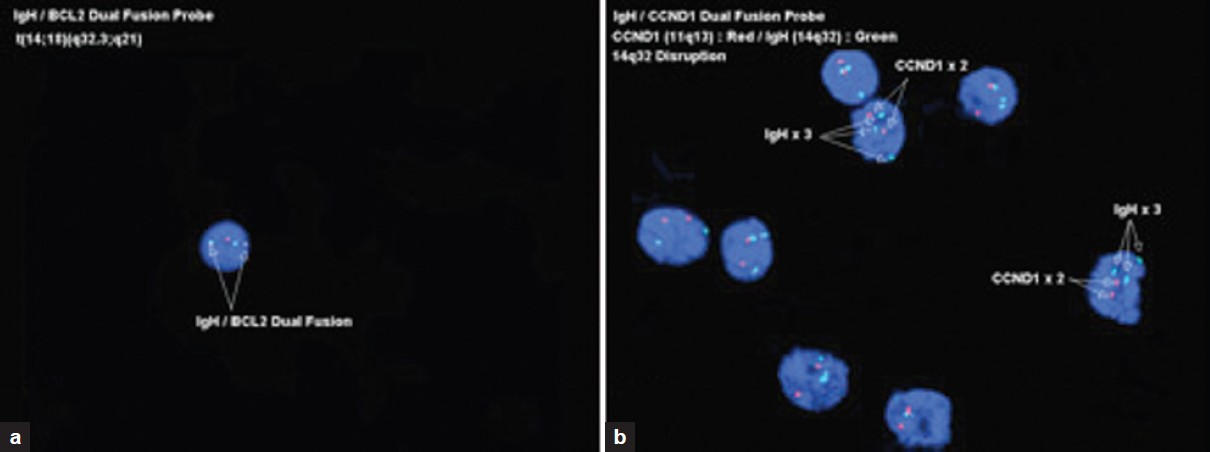
- Fluorescence in situ hybridization performed on Diff-Quik smears, a) A case of FCC showing a positive IGH/BCL2 fusion for the t(14;18) (q32;q21), b) A case of mantle cell lymphoma demonstrating IGH gene rearrangement but with no evidence of IGH/CCND1 fusion
DISCUSSION
Endoscopically-guided FNA procedures (EUS and EBUS) are on the rise, especially for the diagnosis of primary and metastatic lesions. This has been increasingly noted for lung, esophageal, and pancreatic carcinomas. Our study, which analyzed 1 338 such cases of EUS and EBUS-FNAB of deep-seated lymph nodes, is a reflection of this trend. Not only does the large number of cases available to us for analysis speak to the increased popularity of this diagnostic modality, it also supports the observation that this is a relatively non-invasive procedure in staging malignancies. This technique significantly helps clinical colleagues manage their patients.
Determining a definitive diagnosis of lymphoma by aspiration cytology is particularly important for patients whose condition may be too unstable to undergo general anesthesia and open surgical biopsy, a procedure generally associated with a higher risk. Our earlier studies, along with data from other investigators, have demonstrated that endoscopically-guided FNAB is a safe and effective procedure without significant complications. It also is less costly than other methods of obtaining tissue for diagnosis, including mediastinoscopy, thoracotomy, and even computed tomography-guided biopsies.[17] In addition, EUS-FNAB allows access to deep-seated lymph nodes and allows sampling of lesions that are small (<25 mm), and may be difficult to sample using other techniques.[14] Our results suggest that EUS-FNAB is a highly specific and very sensitive technique in the diagnosis of either primary or recurrent lymphoma. In association with flow cytometry and/or immunohistochemical analysis, EUS-FNAB is sensitive (89%) and 100% specific in the diagnosis of primary and recurrent deep-seated NHL and Hodgkin lymphoma.
Our study supports previous literature that the majority of patients who receive a diagnosis by EUS-FNAB will not undergo surgical excisions as confirmatory follow-up.[41218] In our study, 89% of the patients with deep-seated lymphoma were given a definitive cytologic diagnosis. Five patients (11%) required tissue diagnosis including two of which were ultimately given a diagnosis of Hodgkin lymphoma. Thus, in the majority of these cases, treatment decisions were made based on cytopathologic diagnoses and ancillary studies performed on the FNA specimens.
It should be noted that providing a diagnosis of Hodgkin lymphoma via FNA samples alone is inherently challenging. In the one case that we successfully diagnosed, the patient had a prior diagnosis of Hodgkin lymphoma and a significant number of large atypical Reed-Sternberg cells were present in the aspirate. This clinical information in combination with the cytomorphology prompted immunohistochemical staining on the cell block which confirmed the diagnosis. However, in many cases, including the two false negatives seen in this study, the specific clinical history is not known and the yield of large atypical cells that would suggest the diagnosis are not present. In such instances, relatively new, less-invasive modalities such as EUS-guided Trucut biopsy can provide a more informative sample. This allows for a needle core biopsy to be obtained at the time of ICE that can function in a complementary manner to EUS-FNAB by providing tissue architecture and improved cellular yield.[19] In an earlier study, EUS Trucut biopsy provided a diagnosis in six of eight patients where EUS had failed to provide adequate information.[20]
Earlier studies have suggested that FNA may not be adequate in diagnosing lymphomas and perhaps may even mislead therapy.[13] It is generally accepted that excisional biopsies of lymph nodes is a preferred sample to FNA biopsies since it preserves tissue architecture and can provide adequate samples for immunophenotyping and FISH analysis. In this study, we show that EUS-FNA can be effectively utilized to provide a diagnosis of lymphoma (primary or recurrent) and that up to two additional passes can provide an adequate number of cells for flow cytometric analyses. In keeping with earlier observations, we demonstrate here, through the analysis of a large cohort of patient samples, that EUS-FNA is a very sensitive, specific, and accurate modality for the diagnosis of primary and recurrent lymphoma.[131821] Determination of the immunophenotypic profile can delineate in which subclassification (e.g., follicular center cell, mantle cell, and small cell) a low-grade lymphoma belongs, since this is not possible based solely on cytomorphologic features.
In comparison with excisional biopsy, a major limiting factor of FNAB in the diagnosis of lymphoma has been specimen adequacy. Sufficient numbers of viable cells are not only needed for cytologic smears used for rapid interpretation, but also for flow cytometric and/or immunophenotypic analysis.[11] There is sparse literature regarding the cellular yield obtainable by EUS-FNAB and the number of passes required to reach flow cytometric specimen adequacy. Our results show that after two to three passes, the total yield of viable cells per case diagnosed as lymphoma by flow cytometry ranged from 0.12 million to 62.32 million cells (mean, 5.66 million cells). Full flow cytometry panels were performed on a subset of the cases (25, 62.5%). Only three of the 43 cases of NHL submitted for flow cytometry yielded a quantity of cells insufficient for diagnosis. We recommend requesting two dedicated passes for flow cytometric analysis as the viable cellular yield obtained per site and grade of lymphoma can vary greatly.
Cytogenetic and molecular studies can be a valuable tool to aid in the subclassification of NHL, especially when the cellular yield from a specimen is low. Our results show that molecular cytogenetic analysis can be successfully performed on stained and mounted smears from EUS-FNAB specimens, although four specimens did not provide successful results. Successful performance of FISH analysis on recent cases confirmed one case of FCC and demonstrated IGH gene rearrangement in one case of mantle cell lymphoma. Prior studies have demonstrated that archival material can be utilized to perform FISH analysis on cytology specimens, both of which utilized destained Papanicolaou-stained smears.[2223] Bentz and colleagues also included destained Diff-Quik-stained smears. However, they neither expand on the number of Diff-Quik smears nor on the time lag between diagnosis and performance of FISH analysis on these direct smears. We show that FISH analysis on destained Diff-Quik slides can provide supportive evidence of lymphoma in a small subset of cases. Our results also suggest that a shorter time period between collection of the EUS-FNA sample and FISH analysis could improve success rates.
One major challenge of diagnosing lymphomas on FNABs is the cellular yield needed for ancillary studies. One way to increase cellular yield is to request one to two additional passes be performed by the endoscopist to be dedicated for flow cytometric analysis. With the recent addition of 8-color flow cytometry to our laboratory, it is expected that a diagnosis of lymphoma will be rendered on fewer cells, thus making EUS and EBUS-FNAB an increasingly viable and attractive diagnostic tool.
A major criticism of diagnosing lymphoma from FNAB specimens is that architectural patterns are lost through aspiration. Some authors state that adequate grading of follicular center cell lymphoma is not possible on cytologic material.[13] Gong et al.[9] proposed criteria for grading FCC based on the proportion of immunoblastic cells present in the aspirated sample. We did not attempt to grade our cases of deep-seated FCC. When the question of possible transformation of a low-grade lymphoma or a suspected recurrence is at stake, sample adequacy may be more important than histologic pattern.[24] Importantly, we were able to subclassify low-grade lymphomas (five cases of FCC, seven cases of small cell lymphoma, one case of MALToma, and one case of mantle cell lymphoma) based on cytomorphologic and flow cytometric features alone.
Another criticism of the use of FNAB to diagnose deep-seated lymphoma has been its inability to differentiate large cell transformation of FCC from diffuse large B-cell lymphoma. However, because FCC with transformation typically is treated with the same combination chemotherapy used for diffuse large B-cell lymphoma and T-cell rich large B-cell lymphoma, differentiating these two entities has little clinical impact for the patients.[24–27] Liu et al.[28] successfully classified nine of 10 cases of NHL by FNAB, which all had surgical biopsy confirmation. The only discrepant case was one that was diagnosed as FCC on FNAB with the subsequent histologic diagnosis as FCC with focal large cell transformation. In this case, the excisional biopsy was performed at a different site 4 months after the original cytologic diagnosis was made. The authors concluded that this case represented transformation or variable histologic features at different locations. Thus, sampling issues continue to be a challenging reality of FNABs and definitive diagnoses of NHL.
Although grading of follicular NHL has a prognostic role, it seems that clinical factors might have a more significant role in survival than lymphoma subtyping. In a clinical evaluation of the International Lymphoma Study Group classification of NHL, histologic type and patient characteristics as defined by the International Prognostic Index (e.g., age, lactate dehydrogenase level, performance status, and extranodal involvement) predicted patient survival within the NHL subtypes. The study demonstrated significantly different outcomes in terms of overall survival and failure-free survival in an NHL subgroup based on patient clinical characteristics.[24]
Our experience from the present study indicates that the majority of patients who undergo EUS or EBUS-FNAB of deep-seated lymph nodes will not undergo subsequent surgical lymph node excision. When cytomorphologic studies were used in combination with ancillary studies, we were able to provide accurate diagnoses on EUS and EBUS-FNAB samples from deep-seated lymph nodes with high sensitivity and specificity. Our results underscore the importance of this diagnostic modality in patient management. We also show that FISH analysis can be successfully performed on stained and mounted smears from EUS-FNAB specimens.
EUS and EBUS-FNAB used in conjunction with flow cytometry and/or immunohistochemical analysis is a safe, cost-effective, sensitive, and specific method for the diagnosis of primary and recurrent deep-seated lymphoma.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
No competing interest to declare by any of the authors.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author acknowledges that this final version was read and approved. All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) (or its equivalent) of all the institutions associated with this study. Authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2012/9/1/14/95845.
REFERENCES
- Role of endoscopic ultrasound-guided fine-needle aspiration with flow cytometry to diagnose lymphoma: a single center experience. J Gastroenterol Hepatol. 2009;24:1826-33.
- [Google Scholar]
- Endoscopic ultrasound-guided fine-needle aspiration.A cytopathologist′s perspective. Am J Clin Pathol. 2003;120:351-67.
- [Google Scholar]
- Diagnosis of deep-seated lymphoma and leukemia by endoscopic ultrasound-guided fine-needle aspiration biopsy. Am J Clin Pathol. 2006;125:703-9.
- [Google Scholar]
- Endoscopic ultrasound-guided fine-needle aspiration of lymph nodes: the Hennepin County Medical Center experience. Diagn Cytopathol. 2004;30:301-6.
- [Google Scholar]
- Endoscopic ultrasound-guided fine-needle aspiration biopsy: a study of 103 cases. Cancer. 2002;96:232-9.
- [Google Scholar]
- Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: the M. D. Anderson Cancer Center experience. Cancer. 2002;96:174-180.
- [Google Scholar]
- Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA): experience of an academic centre in the USA. Cytopathology. 2010;21:35-43.
- [Google Scholar]
- Fine needle aspiration is a feasible and accurate technique in the diagnosis of lymphoma. J Clin Oncol. 2005;23:9029-30.
- [Google Scholar]
- Fine-needle aspiration in non-Hodgkin lymphoma: evaluation of cell size by cytomorphology and flow cytometry. Am J Clin Pathol. 2002;117:880-8.
- [Google Scholar]
- The accuracy of combined cytopathologic and flow cytometric analysis of fine-needle aspirates of lymph nodes. Am J Clin Pathol. 2000;114:18-28.
- [Google Scholar]
- Fine-needle aspiration cytopathology in diagnosis and classification of malignant lymphoma: accurate and reliable? Diagn Cytopathol. 2000;22:120-5.
- [Google Scholar]
- Utilization of fine-needle aspiration cytology and flow cytometry in the diagnosis and subclassification of primary and recurrent lymphoma. Cancer. 1998;84:252-61.
- [Google Scholar]
- Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J Clin Oncol. 2004;22:3046-52.
- [Google Scholar]
- Endoscopic ultrasound-guided fine-needle aspiration biopsy: a powerful tool to obtain samples from small lesions. Cancer. 2004;102:239-46.
- [Google Scholar]
- Hairy cell leukemia: a diagnosis by endoscopic ultrasound guided fine needle aspiration. Cytojournal. 2006;3:1.
- [Google Scholar]
- Endoscopic ultrasound-guided fine needle aspiration: a powerful modality in the diagnosis of aggressive systemic mastocytosis. Cytopathology. 2011;22:130-2.
- [Google Scholar]
- EUS-guided fine needle aspiration in mediastinal lymphadenopathy of unknown etiology. Gastrointest Endosc. 2002;55:863-9.
- [Google Scholar]
- Diagnosis and subclassification of primary and recurrent lymphoma.The usefulness and limitations of combined fine-needle aspiration cytomorphology and flow cytometry. Am J Clin Pathol. 2000;113:688-99.
- [Google Scholar]
- Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397-401.
- [Google Scholar]
- Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101-6.
- [Google Scholar]
- EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485-91.
- [Google Scholar]
- FISH detection of t(14;18) in follicular lymphoma on Papanicolaou-stained archival cytology slides. Cancer. 2006;108:198-204.
- [Google Scholar]
- Rapid detection of the t(11;14) translocation in mantle cell lymphoma by interphase fluorescence in situ hybridization on archival cytopathologic material. Cancer. 2004;102:124-31.
- [Google Scholar]
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909-18.
- [Google Scholar]
- T-cell-rich B-cell lymphoma: a clinicopathologic study of 21 cases and comparison with 43 cases of diffuse large B-cell lymphoma. Leuk Res. 2004;28:229-36.
- [Google Scholar]
- Follicular lymphoma. In: Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stain H, Thiele J, Vardiman JW, eds. Tumours of the Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. p. :220-6.
- [Google Scholar]
- T-cell-rich B-cell lymphoma.Analysis of clinical features, response to treatment, survival and comparison with diffuse large B-cell lymphoma. Oncology. 2001;61:257-64.
- [Google Scholar]
- Fine-needle aspiration with flow cytometric immunophenotyping for primary diagnosis of intra-abdominal lymphomas. Diagn Cytopathol. 1999;21:98-104.
- [Google Scholar]








