Translate this page into:
Fine needle aspiration cytology of cervical lymph node involvement by ovarian serous borderline tumor
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Serous borderline tumor (SBT) involving a cervical lymph node is extremely rare. In addition, fine needle aspiration (FNA) cytology of the involved cervical lymph node shares tremendous morphologic similarity with other low-grade papillary carcinomas. Thus, it can be easily misdiagnosed as metastatic carcinoma. A 42-year-old female had a history of bilateral SBT and postbilateral salpingo-oophorectomy. She presented with left cervical lymphadenopathy 6 months later. FNA cytology showed a low-grade papillary neoplasm with psammoma bodies. Needle core biopsy along with immunostains was diagnostic of cervical lymph node involvement (LNI) of SBT. although extremely rare, cervical LNI can be found in patients with SBTs. FNA cytology, sometimes, is indistinguishable from metastatic papillary adenocarcinoma. Cell block or needle core biopsy is essential to make the correct diagnosis.
Keywords
Cervical lymph node
fine needle aspiration biopsy
ovarian serous borderline tumor
INTRODUCTION
Serous borderline tumors (SBT) account for 10–15% of ovarian serous tumors.[1] They may occur at any age but are most common in the reproductive era. Most SBTs are Stage I.[2] SBT spread within the pelvis (Stage II) or spread to the abdomen or lymph nodes (Stage III) are not uncommon.[345] Rare Stage IV tumors (<1%) have been reported with cervical lymph node involvement (LNI).[6] Nevertheless, the overall 10-year survival of SBT is excellent, ranging from 65% (Stage II–Stage IV) to 98% (Stage I).[378910]
Although rare, SBTs involving a cervical lymph node are sometimes encountered in the fine needle aspiration (FNA) cytology. However, the cytomorphology of cervical lymph node involved by SBT has not been well-characterized in cytology literature.
Here, we describe a case of FNA cytology of cervical LNI by SBT, presenting as cervical lymphadenopathy, 6 months following bilateral salpingo-oophorectomy of both ovaries.
CASE REPORT
A 42-year-old gravida 0 female experienced several months of pelvic pain and vaginal discharge at the end of 2014. Vaginal ultrasound revealed bilateral adnexal masses and her serum CA125 was elevated (429 U/mL). She subsequently underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, bilateral pelvic, and periaortic lymphadenectomy at an outside institution. The outside pathology, which was confirmed by two of the authors (LC and DAB) revealed bilateral ovarian SBT with noninvasive implants of the omentum and peritoneum. Two of the six bilateral pelvic lymph nodes were involved by SBT. The SBT did not show any micropapillary pattern or areas of microinvasion. The final stage for the SBT was stage IIIC.
Six months later, the patient serum CA 125 was 106 U/mL. A surveillance imaging (Positron emission tomography-computed tomography [PET CT]) revealed left cervical lymphadenopathy. Subsequent ultrasound of the thyroid revealed a small (5 mm) nodule with possible microcalcifications within the left mid pole. The possibility of metastatic thyroid carcinoma was raised. An ultrasound-guided FNA as well as needle core biopsy of the left neck level IV lymph node was performed.
Six cytology smear slides were prepared and subsequently stained with Papanicolaou (Pap) stain. The needle core biopsy was processed by paraffin-embedded block, and the slides were stained with H and E. The Pap-stained smear slides showed numerous papillary groups of epithelioid cells in a background of lymphoid cells [Figure 1]. Psammomatous calcifications were also noted. On high power magnification (×400), the epithelioid cells were intermediate to large as compared to the background lymphocytes but relatively uniform. The cells had a high nuclear-to-cytoplasmic ratio. The nuclei were predominantly oval and exhibited fine nuclear chromatin. Prominent nuclear grooves and irregular nuclear membrane were also observed [Figure 2]. Psammomatous calcifications were also noted [Figure 3]. Definite cytoplasmic intranuclear pseudoinclusions were not seen. Because of the papillary groups of epithelioid cells, the psammomatous calcification, and the nuclear grooves, the diagnosis of metastatic papillary thyroid carcinoma (PTC) was entertained. The needle core biopsy showed extensive deposits of tubular and papillary groups of tumor cells with psammomatous calcification in the background of lymphoid tissue [Figure 4]. The tumor cells were immunoreactive for estrogen receptor (ER) and WT-1 [Figures 5 and 6], whereas they were nonreactive for thyroid transcription factor-1 (TTF-1) and thyroglobulin. Therefore, the diagnosis of cervical lymph node involved by SBT was made.
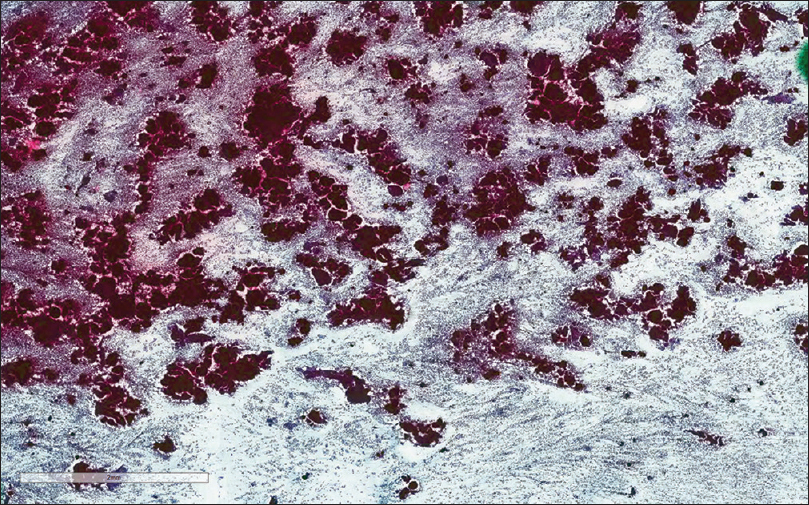
- The Papanicolaou stain cytology smear slide shows numerous papillary groups of epithelioid cells in a background of lymphoid cells (×100)
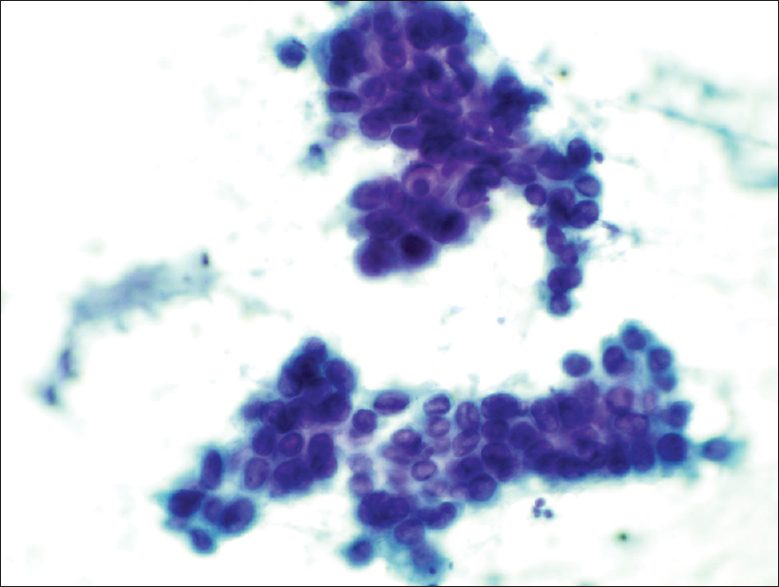
- The Papanicolaou stain cytology smear slide shows that the epithelioid cells were relatively uniform. The cells had a high nuclear-to-cytoplasmic ratio. The nuclei were predominantly oval and exhibited fine nuclear chromatin. Quite prominent nuclear grooves and irregular nuclear membrane were also noted (×400)
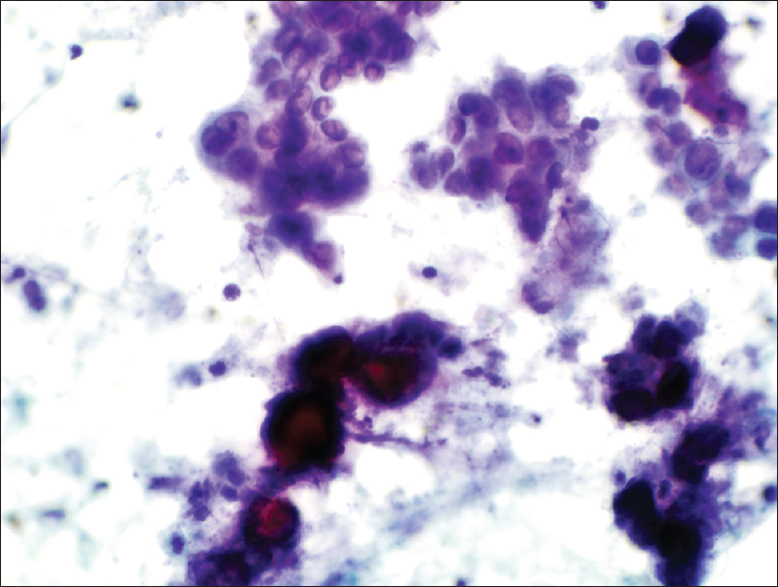
- The Papanicolaou stain cytology smear slide shows several psammoma bodies associated with tumor cells (×400)
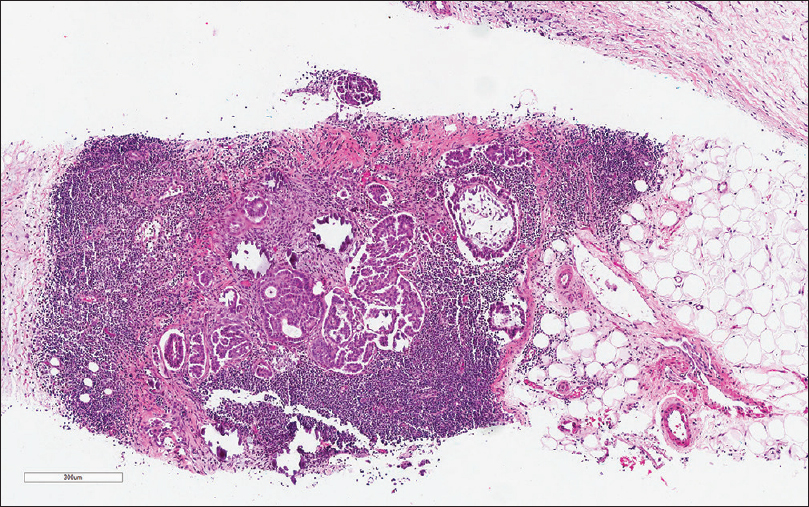
- The needle core biopsy shows deposits of tubular and papillary groups of tumor cells with psammomatous calcification in the background of lymphoid tissue (H and E, ×200)
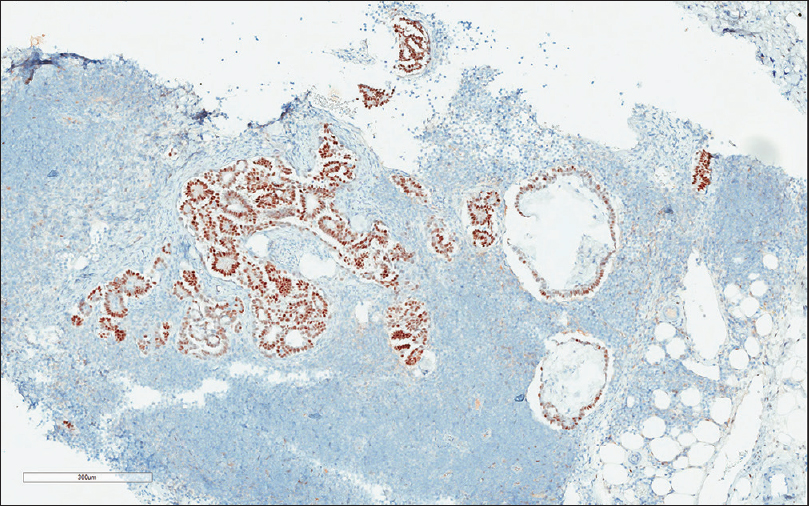
- The tumor cells are immunoreactive with estrogen receptor (×200)
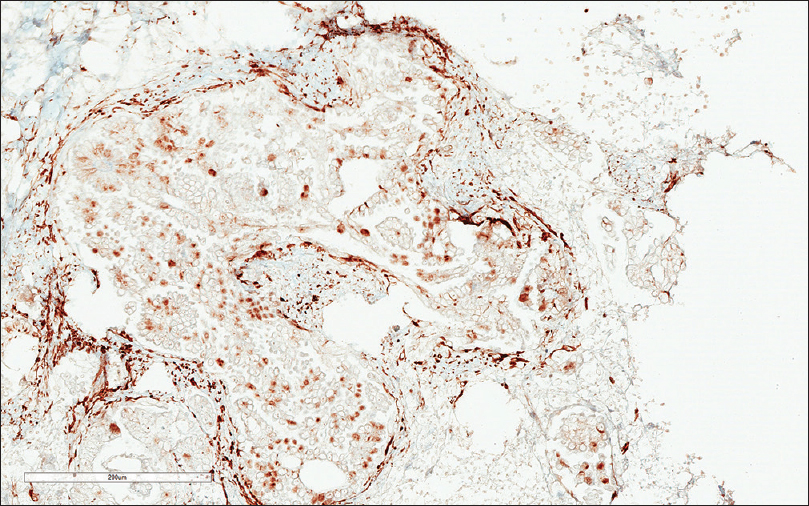
- The tumor cells are immunoreactive (nuclear staining) with WT-1 (×200)
The patient felt very well with no symptoms despite her abdomen and neck lymphadenopathy. Given the low-grade nature of her tumor, a rather conservative management plan including observation and hormonal treatment (aromatase inhibitor) was offered. A subsequent PET-CT found that the patient’s cervical lymphadenopathy is stable.
DISCUSSION
LNI is present in 20–30% of patients with SBT, who undergo lymphadenectomy as part of staging.[4111213] However, supradiaphragmatic LNI is extremely rare.[4] Cervical lymph node by SBT has only been described in the surgical pathology literature by few case reports.[6] To the best of our knowledge, the FNA cytology of cervical LNI by SBT has not been well-reported in cytology literature.
Here, we present this rare FNA cytology case of cervical lymph node involved by SBT. Due to a suspicious 5 mm thyroid nodule detected by ultrasonography, the diagnosis of metastatic thyroid carcinoma is raised clinically. The FNA cytology of this case shows many morphological features overlapping SBT and PTC, which include papillary clusters of epithelial cells, psammoma bodies, and nuclear grooves. Although psammoma bodies are highly characteristic, they are not pathognomonic of PTC and present in only a minority of cases in FNA cytology.[14] One of the major diagnostic features for PTC, cytoplasmic intranuclear pseudoinclusions,[15] is missing in our case. However, the cytological diagnosis of PTC is based on a plethora of morphological features rather than a single feature and the presence of cytoplasmic intranuclear pseudoinclusions is not specific for PTC.[1617] Due to these overlapping features of SBT and PTC, a cell block or needle core biopsy along with immunohistochemical stains are essential to make the correct diagnosis. In this case, we select a panel of ER, TTF-1, thyroglobulin, and WT-1 to differentiate SBT from PTC, since most of the SBTs are positive for ER and WT-1, and they are negative for TTF-1 and thyroglobulin.[1819] These results prove the importance of immunostains in the diagnosis of cervical lymph node involved by SBT.
Because of the low-grade appearance of the tumor cells, we do not consider the possibility of high-grade papillary serous carcinoma metastatic to cervical LN. Another diagnostic consideration is metastatic papillary adenocarcinoma of other sites. The low-power microscopic examination shows numerous papillary groups of epithelial cells. This finding certainly raises the possibility of papillary carcinoma other than PTC, such as lung papillary adenocarcinoma or papillary renal cell carcinoma (RCC). Papillary adenocarcinoma of the lung is composed of true papillae with fibrovascular cores and lined by epithelial cells with nuclear changes reminiscent of PTC.[20] In addition, both lung adenocarcinoma and PTC show the same staining positivity pattern with TTF-1. The immunoprofile of our case excludes the possibility of both tumors.
FNA cytology of type 1 papillary RCC often displays papillae lined by a single layer of uniform epithelial cells. Nuclear grooves, intranuclear pseudo-inclusions, and psammoma bodies may occur and can be indistinguishable from PTC. However, papillary RCC usually shows moderate amount of fine to vacuolated cytoplasm and foamy macrophages.[21] The immunoprofile of ER and WT-1 positivity also excludes the possibility of papillary RCC.
The correct diagnosis of cervical lymph node involved by SBT is paramount for the patient’s clinical management. In our case, if the erroneous diagnosis of metastatic PTC was made, the patient would have a total thyroidectomy. Verbruggen et al. have described three cases of ovarian SBT involving cervical lymph nodes. The preliminary diagnosis of metastatic adenocarcinoma was made on all three cases and resulted in erroneous therapies in one case.[6] Thus, when FNA cytology of cervical lymph nodes showing features of possible papillary adenocarcinoma, it is recommended to include SBT in the differential diagnosis, especially when patients have a clinical history of SBT.
The clinical implication of lymph node involved by SBTs is also intriguing. Most studies have found that LNI is not an adverse prognostic factor independent of stage.[249132223] However, McKenney et al. found that one pattern, >1 mm nodular confluent involvement without intervening lymphoid tissue, may be an adverse prognostic factor.[5] Furthermore, in about 80% of cases of SBT involving lymph nodes, peritoneal implants are also present requiring upstage.[4724] Despite these conflicting data, the clinical management of patients with LNI is generally conservative due to the indolent behavior of SBT.[3912] Cervical lymph node involved by SBT is extremely rare. In cases described by Verbruggen et al., all 3 patients remain free of disease after a follow-up period of 48–84 months. The study concludes that the prognosis of cervical lymph node involved by SBT differs significantly from that of adenocarcinoma of unknown primary; therefore, the correct diagnosis is critical to avoid ineffective treatment with chemotherapy.[6]
In summary, although rare, cervical LNI can be found in patients with SBT. FNA cytology of these positive lymph nodes, sometimes, is indistinguishable from metastatic papillary adenocarcinoma. Cell block or core biopsy along with immunocytochemical stains are essential to make the correct diagnosis.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors have no competing of interest to declare.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author (LC, KAB, and DAB) has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
The study subject has given her informed consent, and the information suitable to reveal the subject’s identity is to be avoided.
LIST OF ABBREVIATIONS (In alphabetic order)
ER - Estrogen Receptor
FNA - Fine Needle Aspiration
LNI - Lymph Node Involvement
Pap - Papanicolaou
PTC - Papillary Thyroid Carcinoma
PET-CT - Positron Emission Tomography-Computed Tomography
RCC - Renal Cell Carcinoma
SBT - Serous Borderline Tumor
TTF-1 - Thyroid Transcription Factor-1
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Serous borderline (low malignant potential, atypical proliferative) ovarian tumors: Workshop perspectives. Hum Pathol. 2004;35:934-48.
- [Google Scholar]
- Ovarian serous borderline tumors: A critical review of the literature with emphasis on prognostic indicators. Hum Pathol. 2000;31:539-57.
- [Google Scholar]
- Comparison of the clinical behavior of newly diagnosed stages II-IV low-grade serous carcinoma of the ovary with that of serous ovarian tumors of low malignant potential that recur as low-grade serous carcinoma. Gynecol Oncol. 2007;105:625-9.
- [Google Scholar]
- Lymph node disorders and prognostic value of nodal involvement in patients treated for a borderline ovarian tumor: An analysis of a series of 42 lymphadenectomies. J Am Coll Surg. 2002;195:332-8.
- [Google Scholar]
- Lymph node involvement in ovarian serous tumors of low malignant potential (borderline tumors): Pathology, prognosis, and proposed classification. Am J Surg Pathol. 2006;30:614-24.
- [Google Scholar]
- Serous borderline tumor of the ovary presenting with cervical lymph node involvement: A report of 3 cases. Am J Surg Pathol. 2006;30:739-43.
- [Google Scholar]
- Serous tumors involving extra-abdominal/extra-pelvic sites after the diagnosis of an ovarian serous neoplasm of low malignant potential. Am J Surg Pathol. 2001;25:988-96.
- [Google Scholar]
- Serous borderline tumors of the ovary: A long-term follow-up study of 137 cases, including 18 with a micropapillary pattern and 20 with microinvasion. Am J Surg Pathol. 2002;26:1111-28.
- [Google Scholar]
- Long-term survival and patterns of care in women with ovarian tumors of low malignant potential. Gynecol Oncol. 2002;86:34-7.
- [Google Scholar]
- Advanced-stage serous borderline tumors of the ovary: A clinicopathological study of 49 cases. Int J Gynecol Pathol. 2003;22:29-36.
- [Google Scholar]
- Ovarian serous borderline tumors with lymph node involvement. Clinicopathologic and DNA content study of seven cases and review of the literature. Am J Surg Pathol. 1994;18:904-12.
- [Google Scholar]
- Ovarian papillary serous tumors of low malignant potential (serous borderline tumors).A long-term follow-up study, including patients with microinvasion, lymph node metastasis, and transformation to invasive serous carcinoma. Cancer. 1996;78:278-86.
- [Google Scholar]
- Lymph node involvement in ovarian serous tumors of low malignant potential: A clinicopathologic study of thirty-six cases. Am J Surg Pathol. 2010;34:1-9.
- [Google Scholar]
- Intranuclear cytoplasmic inclusions in fine-needle aspiration smears of papillary thyroid carcinoma: A study of its morphological forms, association with nuclear grooves, and mode of formation. Diagn Cytopathol. 2005;32:264-8.
- [Google Scholar]
- A step-wise logistic regression analysis of papillary carcinoma of the thyroid. Acta Cytol. 1986;30:285-93.
- [Google Scholar]
- Intranuclear vacuoles in nonpapillary carcinoma of the thyroid. A report of three cases. Acta Cytol. 1984;28:581-6.
- [Google Scholar]
- Quantification of ER/PR expression in ovarian low-grade serous carcinoma. Gynecol Oncol. 2013;128:371-6.
- [Google Scholar]
- Lung adenocarcinoma and its thyroid metastasis characterized on fine-needle aspirates by cytomorphology, immunocytochemistry, and next-generation sequencing. Diagn Cytopathol. 2015;43:585-9.
- [Google Scholar]
- Fine-needle aspiration cytology of papillary renal cell carcinoma: The association with concomitant secondary malignancies. Diagn Cytopathol. 2006;34:797-800.
- [Google Scholar]
- Ovarian serous tumors of low malignant potential (borderline tumors): Outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am J Surg Pathol. 2005;29:707-23.
- [Google Scholar]
- A nationwide study of serous “borderline” ovarian tumors in Denmark 1978-2002: Centralized pathology review and overall survival compared with the general population. Gynecol Oncol. 2014;134:267-73.
- [Google Scholar]
- The recurrence and the overall survival rates of ovarian serous borderline neoplasms with noninvasive implants is time dependent. Am J Surg Pathol. 2006;30:1367-71.
- [Google Scholar]








