Translate this page into:
Greater specificity of p40 compared with p63 in distinguishing squamous cell carcinoma from adenocarcinoma in effusion cellblocks

*Corresponding author: Ji Shin Lee, MD, PhD Department of Pathology, Chonnam National University Medical School, Gwangju, Republic of Korea. jshinlee@hanmail.net
-
Received: ,
Accepted: ,
How to cite this article: Kim NI, Lee JS. Greater specificity of p40 compared with p63 in distinguishing squamous cell carcinoma from adenocarcinoma in effusion cellblocks. CytoJournal 2020;17:13.
Abstract
Objective:
Squamous cell carcinoma (SCC) rarely causes malignant effusions. Distinguishing between SCC and adenocarcinoma in effusion cytology can be a challenge. p63 and p40 have been frequently used to support squamous cell differentiation in both histological and cytological specimens. However, similar results in cytological preparations of effusion fluids have been rarely reported. This study was designed to assess the diagnostic value of p63 and p40 immunoreactivity for the differentiation of SCC from adenocarcinoma in malignant effusions.
Materials and Methods:
Immunocytochemical staining of p63 and p40 was performed on thirty cellblock specimens, including ten malignant effusions carrying SCC and twenty malignant effusions showing adenocarcinoma. Any degree of nuclear staining was considered positive.
Results:
Of the ten SCC cases, 100% tested positive for both p63 and p40, and most cases showed diffuse staining (>25% of tumor cells). The expression of p63 and p40 was detected in 4 (20%) and 2 (10%) of twenty adenocarcinoma cases, and the extent of staining was all focal (≤25% of tumor cells). The p63 reactivity showed 100% sensitivity, 80% specificity, 71% positive predictive value, and 100% negative predictive value for the differentiation of SCC from adenocarcinoma in malignant effusions. The sensitivity of p40 for SCC was 100%, the specificity was 90%, the positive predictive value was 83%, and the negative predictive value was 100%.
Conclusion:
Although p63 and p40 are both useful markers for the diagnosis of SCC in malignant effusions, p40 is more specific than p63 in distinguishing SCC from adenocarcinoma.
Keywords
Cellblocks
Effusion
Immunocytochemistry
p40
p63
Squamous cell carcinoma
INTRODUCTION
Effusion is the accumulation of fluid in body cavities. Effusion has various benign and malignant etiologies.[1] Cytological examination of effusion fluids is a rapid and common analytical method for the determination of the nature (benign or malignant) of effusion. Malignant effusions represent a sign of cancer dissemination and the end stage of the disease. Accurate tumor typing of malignant effusions is important in the era of personalized medicine.[2]
Squamous cell carcinoma (SCC) is one of the most common cancers in the world and the main cause of cancer-related death.[3] However, effusions caused by metastatic SCC are extremely rare.[3-5] The most recent series of SCC involving effusions to date was published in 2017, in which a total of 49 effusions to body cavities were identified in 26 patients with metastatic SCC during a period of 17 years.[5]
The diagnosis of SCC in effusion cytology is generally not difficult in patients with well-known clinical history and characteristic cytomorphological findings.[3-5] However, moderate-to-poorly differentiated SCC in effusions presents a diagnostic challenge owing to its overlapping features with various other conditions, including metastatic adenocarcinoma, the most common cause of malignant effusions.[3-5]
Since the treatment for SCC differs from that of adenocarcinoma, accurate identification of squamous cell differentiation has become critical, especially in lung specimens.[6-9] Numerous immunohistochemical and immunocytochemical markers have been recently explored for their utility in distinguishing pulmonary SCC and adenocarcinoma. p63 and p40 have been frequently used to differentiate SCC from adenocarcinoma in both histological and cytological specimens.[10-14] Although p63 and p40 have been extensively investigated in the diagnosis of SCC and its differentiation from adenocarcinoma, these markers have infrequently been reported in cytologic preparations of effusion fluids.[3,5,13,15]
Cells exfoliated into effusion fluid can be analyzed as smears, cytospins, liquid-based preparations, and cellblocks. Cellblock is a good method for immunocytochemistry,[1,2] and our laboratory prefers cellblock method that provides intact tissue sections for immune cell chemistry. In our current study, we evaluated the diagnostic value of p63 and p40 immunoreactivity to distinguish SCC from adenocarcinoma in effusion cellblocks.
MATERIALS AND METHODS
Patient selection
A total of ten cases of malignant effusion with SCC were retrieved from the pathology database at Chonnam National University Hwasun Hospital between 2015 and 2018 when a total of 4867 serous fluids were collected. Malignant effusions with SCC were diagnosed based on cytological features, clinical history, corresponding cellblock material, and imaging studies. Twenty cases of malignant effusions with adenocarcinoma were also retrieved. The primary site in all cases was confirmed by surgical biopsy or resection. Some adenocarcinoma cases were offered by the Biobank of Chonnam National University Hwasun Hospital, a member of the Korea Biobank Network. This retrospective study utilized archived materials and did not impact patient care; hence, it was granted our hospital Institutional Review Board approval without patient consent (reference: CNUHH-2019-043).
ThinPrep and cellblocks
For each case, ThinPrep and formalin-fixed paraffin-embedded cellblocks were prepared as previously described.[16]
Immunocytochemistry
Only cellblocks with adequately preserved diagnostic material were selected for immunocytochemistry. Immunocytochemical staining was performed on a Bond-Max automated immunostainer (Leica Microsystems, Bannockburn, IL, USA) as previously described.[17] Briefly, all steps were performed using the automated instrument according to the manufacturer’s instructions, using the following primary antibodies: anti-p63 (1:50 dilution, clone 4A4, Dako, Carpinteria, CA, USA) and anti-p40 (1:200 dilution, rabbit polyclonal, Diagnostic BioSystems, Pleasanton, CA, USA).
The stained slides were independently evaluated by two cytopathologists unaware of the diagnosis. Any degree of nuclear staining was considered positive for p63 and p40. In addition, the extent of staining was semi-quantitatively estimated as follows: 0, absent staining; 1+, <5%; 2+, 6%– 25%; 3+, 26%–75%; and 4+, >76%.[13] In addition, we used 25% expression as a cutoff to distinguish between focal and diffuse staining.
Statistical analysis
Analyses of the differences between variables were performed using the Chi-square or Fisher’s exact test. The P values for the comparison of the extent of immunoreactivity were determined through a two-tailed Mann–Whitney test. Differences were considered statistically significant only when P < 0.05.
RESULTS
The ten patients with metastatic SCC included eight males and two females, with an average age of 62 years. The malignant effusions comprised eight pleural fluids (80%), one peritoneal fluid (10%), and one pericardial fluid (10%). The most common primary site of SCC was lung (50% of cases), followed by the uterine cervix (20%). There were 4 (40%) keratinizing and 6 (60%) nonkeratinizing SCCs. Of the twenty malignant effusions with metastatic adenocarcinoma obtained, ten were pleural and ten peritoneal fluids. Lung and ovary were the most common primary sites associated with pleural and peritoneal effusion, respectively.
Immunocytochemical results are summarized in Table 1. The expression of p63 and p40 was demonstrated in the nuclei of tumor cells. Of the ten SCC cases, 100% were positive for both p63 and p40 [Figure 1]. Although most cases showed diffuse staining (>25% of tumor cells) for both p63 and p40, the extent of staining for p63 was higher than that of p40 (P < 0.05) [Table 1 and Figure 2].
| Extent | p63 | p40 | ||
|---|---|---|---|---|
| Malignant effusions with metastatic SCC (n=10), n(%) | Malignant effusions with metastatic ADC (n=20), n(%) | Malignant effusions with metastatic SCC (n=10), n(%) | Malignant effusionswith metastatic ADC (n=20), n(%) | |
| Negative | 0 | 16 (80) | 0 | 18 (90) |
| Positive | 10 (100) | 4 (20) | 10 (100) | 2 (10) |
| <5% | 0 | 3 (75) | 0 | 1 (50) |
| 6-25% | 1 (10) | 1 (25) | 4 (40) | 1 (50) |
| 26-75% | 7 (70) | 0 | 6 (60) | 0 |
| >76% | 2 (20) | 0 | 0 | 0 |
SCC: Squamous cell carcinoma, ADC: Adenocarcinoma

- Metastatic oral squamous cell carcinoma in pleural effusion. Small orangeophilic squamous-like cells present in liquid-based cytology (a) and cellblock sections (b) (a, Papanicolaou, ×400; b, H and E, ×400). Nuclear immunostaining for p63 (c) and p40 (d) is evident (c and d, ×400).
- Metastatic pulmonary squamous cell carcinoma in pleural effusion. Tight cell cluster wrapped by elongated and flattened cells is seen (a, Papanicolaou, ×400). Squamous eddy is characteristic of cellblock section (b, H and E, ×400). p63 (c) and p40 (d) are strongly and diffusely positive (c and d, ×400). The extent of p63-positive staining is higher than that of p40.
The expression of p63 and p40 was detected in 4 (20%) and 2 (10%) of twenty cases of malignant effusion with adenocarcinoma, respectively, and the extent of staining was all focal (≤25% of tumor cells) [Figures 3 and 4]. Two cases of adenocarcinoma were positive for both p63 and p40. The differences in immunostaining between SCC and adenocarcinoma for both p63 and p40 in malignant effusions were statistically significant (P < 0.001 for both). The extent of p63 and p40 expression in SCC was higher than in adenocarcinoma (P < 0.001 for both).

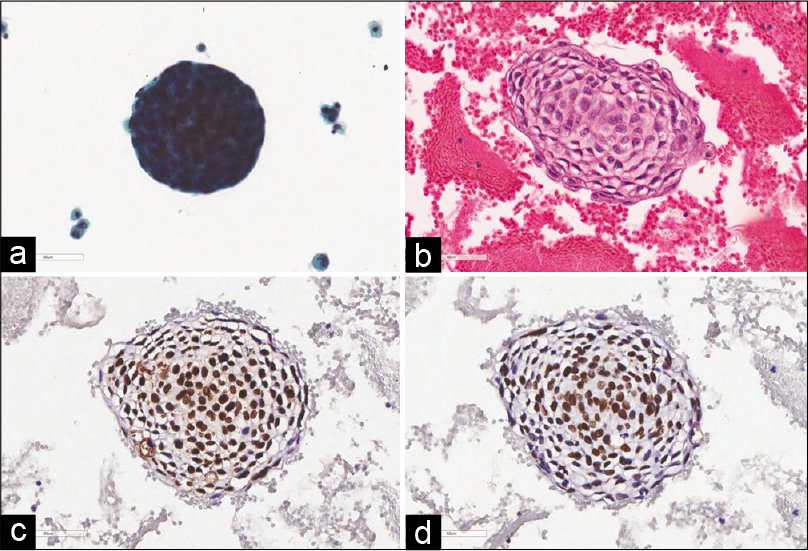
- Metastatic ovarian adenocarcinoma in peritoneal effusion. The malignant cells form three-dimensional balls (a, Papanicolaou, ×400). Clear-cut glandular differentiation is detected in cellblock section (b, H and E, ×400). p63 (c) and p40 (d) immunostaining are observed in several cells (c and d, ×400).

- Metastatic pulmonary adenocarcinoma in pleural effusion. Cell cluster with irregular border is present in liquid-based cytology (a) and cellblock section (b) (a, Papanicolaou, ×400; b, H and E, ×400). p63 (c) and p40 (d) are completely negative (c and d, ×400).
Table 2 summarizes the sensitivity and specificity of immunostaining with p63 and p40. The p63 reactivity was found to be 100% sensitive and 80% specific with a positive predictive value of 71% and a negative predictive value of 100% for distinguishing SCC from adenocarcinoma. The sensitivity, specificity, positive predictive value, and negative predictive value of p40 for distinguishing SCC from adenocarcinoma were 100%, 90%, 83%, and 100%, respectively; p40 was more specific than p63 in distinguishing SCC from adenocarcinoma (P < 0.05).
| Diagnostic utility | p60 (%) | p40 (%) |
|---|---|---|
| Sensitivity | 10/10 (100) | 10/10 (100) |
| Specificity | 16/20 (80) | 18/20 (90) |
| Positive predictive value | 10/14 (71) | 10/12 (83) |
| Negative predictive value | 16/16 (100) | 18/18 (100) |
Positive predictive value: True positive/(true positive + false positive), Negative predictive value: True negative/(true negative + false negative)
DISCUSSION
In the current study, we evaluated the efficacy of p63 and p40 markers for distinguishing SCC from adenocarcinoma in malignant effusions. We showed that p63 and p40 were both very sensitive markers for the diagnosis of SCC in effusions. Moreover, p40 was more specific than p63 in differentiating SCC from adenocarcinoma.
Effusions occur in a variety of benign and malignant diseases, and malignant effusions are a sign of end stage of cancer.[1] Cytologic evaluation of effusion fluids is a quick and accurate method to diagnose metastatic disease. Metastatic adenocarcinoma of various origins is the most common tumor encountered in malignant effusions.[2]
Although SCC is one of the most common cancers worldwide, malignant effusions with SCC are extremely rare.[3-5] According to a literature review, the most recent series of malignant effusions associated with SCC was published in 2017, in which a total of 49 fluids from 26 patients were identified during a period of 17 years.[5] Pulmonary SCC was common (65%), followed by head and neck (16%). Huang and Michael found only 36 cases of SCC (0.2%) in 18,626 serous fluids over a period of 22 years.[3] The lung and head and neck were the major primary sites of metastasis to the pleura and pericardium, respectively, and the uterine cervix was the most common cause of intraperitoneal metastasis. Smith-Purslow et al. reported 46 (0.5%) patients with metastatic SCC to pleural, peritoneal, or pericardial cavities in 9297 serous effusions over a period of 33 years.[4]
Only 10 (0.2%) out of 4867 cases of serous fluids were identified in our pathology database over a period of 4 years. Eight were pleural fluids, one was peritoneal fluid, and one was pericardial fluid. The most common primary site of SCC was lung (50%), followed by the uterine cervix (20%). Our results are consistent with previous studies.[3-5]
The diagnosis of malignant effusions due to SCC is usually not a problem when the patient’s clinical history is established and based on the characteristic cytomorphology, especially the presence of keratinized cells and squamous eddies.[3-5] However, if the SCC in effusions is less differentiated, the diagnosis can be difficult and is easily misclassified.
With the evolution of personalized medicine, it is becoming increasingly important to accurately identify squamous differentiation in cytology specimens.[2] This issue is best exemplified in both surgical and cytological specimens of the lung, in which the distinction between SCC and adenocarcinoma is crucial for management using novel targeted therapies.[6-9] Despite the clear need for pathological and cytopathological differentiation of SCC from pulmonary adenocarcinoma accurately, the morphological distinction between SCC and adenocarcinoma is difficult. Immunohistochemistry and immunocytochemistry have recently emerged as useful ancillary tools in resolving this dilemma. Numerous markers are generally used to distinguish SCC from adenocarcinoma of the lung.[2]
The most commonly used marker to establish squamous differentiation is p63, which recognizes all six isoforms of the p63 protein.[10] Another antibody commonly employed to identify SCC is p40, which only recognizes the three ΔNp63 isoforms.[10] Many studies have evaluated the clinical performance of p63 and p40 for distinguishing SCC from adenocarcinoma in both surgical and various cytological samples.[10-15] These studies found that both p63 and p40 showed high sensitivities (approaching 100%) for the detection of pulmonary SCC. They also reported superior specificity using p40 than p63, with a higher rate of false positivity for p63 of up to 63% in adenocarcinomas compared with 0%–21% for p40.[10-14]
Although p63 and p40 have been extensively studied in the differentiation SCC from adenocarcinoma, few studies have evaluated the role of p63 and p40 immunocytochemistry in cytological preparations of effusion fluids.[3,5,13,15] The cancer cells in effusion fluids may have altered profiles of protein expression because of different molecular signaling in the environment.[18,19] Thus, it is necessary to perform a validation study in order to extend the use of p63 and p40 in effusion cytology. Similarly, p63 initially showed promise in distinguishing SCC from adenocarcinoma in effusion fluids. Pu et al. evaluated the utility of immunocytochemical panels including p63 in differentiating SCC, adenocarcinoma, and malignant mesothelioma in effusion cellblocks.[15] All of the SCC and adenocarcinoma originated primarily from the lungs. Using 10% nuclear staining without reference to intensity as a cutoff for interpretation, p63 was detected in 12 of 15 (80%) SCCs and 3 of 10 (30%) adenocarcinomas. Huang and Michael used various immunocytochemical panels including p63 on cellblock sections of malignant effusions.[3] p63 reactivity was found in 12 (80%) of 15 SCCs and 3 (13%) of 25 adenocarcinomas.
Recently, LePhong et al. reported 26 patients with malignant effusions with SCC.[5] They performed immunocytochemical staining of cellblock specimens using a variety of markers, including p63 and p40. The p63 and p40 immunoreactivity was found in 6 (67%) of 9 SCC cases and 3 (100%) of 3 SCC cases, respectively. The relatively low positivity of p63 was explained by the scant cellularity of one of the three p63-negative cases and staining of only a few tumor cells in another case. Alexander et al. reported p63 and p40 immunocytochemistry in 190 cellblock specimens derived from fine-needle aspirations and effusions.[13] Semi-quantitative analysis using a monoclonal p63 antibody (clone 4A4) and a rabbit polyclonal p40 antibody was utilized to determine both intensity (0, absent staining; 1, weak staining; 2, moderate staining; and 3, equivalent to positive control) and extent (0, absent staining; 1, 1% to 25%; 2, 25% to 50%; and 3, 50% to 100%) of nuclear staining and calculate the overall score based on cumulative intensity and extent scores. An overall score of 2 or greater was considered positive. All the five effusions with pulmonary SCC tested positive for both p63 and p40, with most cases showing strong intensity. The positive expression of p63 and p40 was found in 3 (15%) and 8 (40%) of 20 effusions with pulmonary adenocarcinoma.
The existing studies focused on cellblock specimens of malignant effusions with SCC or adenocarcinoma and carry small sample sizes (ranging from 3 to 25). For distinguishing SCC from adenocarcinoma in effusion fluids, the sensitivity of p63 ranged from 80% to 100%, whereas the specificity ranged from 70% to 87%.[3,13,15] The sensitivity of p40 was 100%, whereas the specificity was 60%.[13]
In the current study, p63 and p40 immunocytochemical staining was performed on thirty cellblock specimens, including ten malignant effusions with SCC and twenty malignant effusions with adenocarcinoma. Of the ten SCC cases, 100% were positive for both p63 and p40. The expression of p63 and p40 was detected in 4 (20%) and 2 (10%) of twenty cases of adenocarcinoma. The differences in immunostaining for both p63 and p40 between SCC and adenocarcinoma were statistically significant, and p63 and p40 were 100% sensitive for SCC. Furthermore, most cases showed diffuse staining (>25% of tumor cells) for both p63 and p40. Our result supports p63 and p40 as useful markers for the diagnosis of SCC in malignant effusions.
The specificity of p63 and p40 for SCC in effusion fluids was 80% and 90%, respectively, for the differentiation of SCC from adenocarcinoma, suggesting that p40 was a more specific marker than p63. Although limited by the small sample size, the results are consistent with several previous studies reporting a higher specificity of p40 compared with p63.[10-12,14] However, Alexander et al. reported a specificity of 85% for p63, which was higher than 60% for p40.[13] The reasons for this discrepancy are not known; however, they may be explained by differences in preanalytical and analytical variables. The p63 and p40 antibodies we used were the same as those used by Alexander et al.[13] Any degree of nuclear staining was the threshold used to define positive results in the current study, which was equivalent to the cutoff value of Alexander et al.[13] However, we included malignant effusions with various body cavities and primary sites of tumors, whereas the series by Alexander et al. focused on pleural effusions induced by pulmonary SCC or adenocarcinoma.[13] Considering the specificity of p63 and p40 in differentiating SCC from adenocarcinoma, further studies involving larger cohorts of malignant effusions with adenocarcinoma are needed.
Although p63 and p40 are very sensitive markers for SCC, adenocarcinoma showed a positive staining for both. Therefore, both p63 and p40 should be evaluated using a panel of markers specific for adenocarcinoma when SCC and adenocarcinoma are considered in the differential diagnosis of malignant effusions. MUC4, thyroid transcription factor-1, napsin-A, and claudin-4 are good candidates for adenocarcinoma makers.[2,20-22]
CONCLUSION
Metastatic SCC is very rare in serous effusions. Although p63 and p40 are both useful and confirmatory markers for SCC in serous effusions, p40 is more specific than p63 in differentiating SCC from adenocarcinoma in effusion fluids.
Acknowledgments
This study was presented in part at the European Congress of Cytology 2019, Malmö, Sweden, June 16, 2019. This study was supported by a grant (HCRI 19 001-119013) Chonnam National University Hwasun Hospital Institute for Biomedical Science.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All co-authors have read the manuscript and agree for submission.
ETHICS STATEMENT BY ALL AUTHORS
This project was approved by the Institutional Review Board in CNUHH.
LIST OF ABBREVIATIONS (In alphabetic order)
SCC - Squamous cell carcinoma.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (the authors are blinded for reviewers and vice versa) through automatic online system.
References
- The diagnosis of malignancy in effusion cytology: A pattern recognition approach. Adv Anat Pathol. 2006;13:174-84.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers in the diagnosis of pleural diseases: A 2018 update. Ther Adv Respir Dis. 2018;12:1753466618808660.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomorphological features of metastatic squamous cell carcinoma in serous effusions. Cytopathology. 2014;25:112-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cells of squamous cell carcinoma in pleural, peritoneal and pericardial fluids. Origin and morphology. Acta Cytol. 1989;33:245-53.
- [Google Scholar]
- Squamous cell carcinoma in serous effusions: Avoiding pitfalls in this rare encounter. Diagn Cytopathol. 2017;45:1095-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339-46.
- [CrossRef] [PubMed] [Google Scholar]
- Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693-703.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6:64-70.
- [CrossRef] [PubMed] [Google Scholar]
- Current concepts on the molecular pathology of non-small cell lung carcinoma. Semin Diagn Pathol. 2014;31:306-13.
- [CrossRef] [PubMed] [Google Scholar]
- P40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol. 2012;25:405-15.
- [CrossRef] [PubMed] [Google Scholar]
- Expressions of thyroid transcription factor-1, napsin A, p40, p63, CK5/6 and desmocollin-3 in non-small cell lung cancer, as revealed by imprint cytology using a malinol-based cell-transfer technique. Acta Cytol. 2015;59:457-64.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of P40 and P63 in lung cancers using fine needle aspiration cases. Understanding clinical pitfalls and limitations. J Am Soc Cytopathol. 2016;5:123-32.
- [CrossRef] [PubMed] [Google Scholar]
- Can p40 (Polyclonal) replace p63 (Clone 4A4) in the cytologic diagnosis of pulmonary non-small cell carcinoma? Am J Clin Pathol. 2017;147:580-8.
- [CrossRef] [PubMed] [Google Scholar]
- P40 immunohistochemistry is an excellent marker in primary lung squamous cell carcinoma. J Pathol Transl Med. 2018;52:283-9.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of WT-1, p63, MOC31, mesothelin, and cytokeratin (K903 and CK5/6) immunostains in differentiating adenocarcinoma, squamous cell carcinoma, and malignant mesothelioma in effusions. Diagn Cytopathol. 2008;36:20-5.
- [CrossRef] [PubMed] [Google Scholar]
- Immunocytochemical panel for distinguishing between adenocarcinomas and reactive mesothelial cells in effusion cell blocks. Diagn Cytopathol. 2009;37:258-61.
- [CrossRef] [PubMed] [Google Scholar]
- Stromal matrix metalloproteinase-14 expression correlates with the grade and biological behavior of mammary phyllodes tumors. Appl Immunohistochem Mol Morphol. 2012;20:298-303.
- [CrossRef] [PubMed] [Google Scholar]
- Altered expression and activation of the nerve growth factor receptors trkA and p75 provide the first evidence of tumor progression to effusion in breast carcinoma. Breast Cancer Res Treat. 2004;83:119-28.
- [CrossRef] [PubMed] [Google Scholar]
- Effusion and solid lymphomas have distinctive gene and protein expression profiles in an animal model of primary effusion lymphoma. J Pathol. 2006;209:464-73.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic usefulness of MUC1 and MUC4 for distinguishing between metastatic adenocarcinoma cells and reactive mesothelial cells in effusion cell blocks. Acta Cytol. 2013;57:377-83.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of thyroid transcription factor-1 and CDX-2 in determining the primary site of metastatic adenocarcinomas in serous effusions. Acta Cytol. 2010;54:277-82.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic usefulness of claudin-3 and claudin-4 for immunocytochemical differentiation between metastatic adenocarcinoma cells and reactive mesothelial cells in effusion cell blocks. Acta Cytol. 2016;60:232-9.
- [CrossRef] [PubMed] [Google Scholar]








