Translate this page into:
Mucinous tubular and spindle cell carcinoma of the kidney: Diagnosis by fine needle aspiration and review of the literature
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Renal mucinous tubular and spindle cell carcinoma (MTSCC) was recently described as a distinct subtype of renal cell carcinoma (RCC) in the 2004 World Health Organization classification of kidney tumors. MTSCC is a rare low grade malignancy with < 100 cases reported in the literature. To the best of our knowledge, there are 5 case reports with a total of 6 patients describing its diagnosis by fine needle aspiration (FNA). All of these cases were diagnosed as conventional RCC on FNA. Subsequent excisions proved them to be MTSCC. We herein report a case in a 67-year-old male. He presented with abdominal pain and was found to have a new colon adenocarcinoma with metastasis to the liver and lungs. The extent of disease made the patient ineligible for surgical excision, and he received chemotherapy. Work-up also revealed a kidney mass which was later biopsied by FNA and core biopsy. The tumor was composed of epithelial and spindled cell components embedded in a myxoid background. It was positive for CK7, AMCAR, vimentin, and epithelial membrane antigen. The tumor was diagnosed as MTSCC. One year later the kidney mass remained stable. However, the patient developed new metastasis to the liver from colonic primary. The kidney mass was not resected. Although rarely encountered in FNA cytology of the kidney, we believe the cytologic features of this tumor are distinctive and are different from conventional and other subtypes of RCC. Therefore, its accurate diagnosis on FNA is possible once pathologists are aware that MTSCC should be considered in the differential diagnosis of kidney tumors.
Keywords
Fine needle aspiration
kidney
renal mucinous tubular spindle cell carcinoma
INTRODUCTION
Renal cell carcinoma (RCC) is comprised a heterogeneous group of epithelial kidney tumors. Each of these subtypes has unique histopathologic, genetic, and clinical features.[1] Renal mucinous tubular and spindle cell carcinoma (MTSCC) was recently described as a distinct subtype of RCC in the 2004 World Health Organization (WHO).[2] MTSCC is a low grade malignancy with a favorable prognosis. It has a female predominance over a broad age range.
MTSCC is a rare tumor. There are <10 cases reported in the cytology literature describing its diagnostic features on fine needle aspiration (FNA).[3] There are overall <100 cases reported to date.[1]
We herein present a case of MTSCC diagnosed by FNA with concurrent core biopsy in an older gentleman; this case is of particular interest as MTSCC was discovered as an incidental finding in the setting of newly diagnosed metastatic colon adenocarcinoma. Although MTSCC has well defined histologic features, its cytologic diagnosis remains challenging with conventional clear cell RCC or RCC without further subclassification being the most commonly rendered diagnosis.
CASE REPORT
A 67-year-old man presented with abdominal pain. Clinical work-up included a computed tomography (CT) scan which revealed a 4.5 cm mass consistent with an ascending colon carcinoma with pericolonic lymphadenopathy, liver, and lung metastases. Also seen was a 3.5 cm left kidney mass. A CT-guided biopsy of a 2.2 cm lesion in the right lower lobe of the lung revealed metastatic adenocarcinoma of colon origin. The kidney lesion was described as a hypoenhancing solid mass. It was felt initially to represent either a metastasis or a primary renal neoplasm. The patient was treated with chemotherapy for metastatic colonic adenocarcinoma and no renal biopsy was performed. About 7 months later, the renal lesion remained unchanged with chemotherapy whereas other metastatic sites decreased in size. CT-guided FNA and concurrent core biopsies were performed at this time.
Cytologic findings
FNA smears were stained with Diff Quik and Papanicolaou stains. Smears were cellular and showed crowded small and large clusters and branching sheets of bland epithelial cells with uniform round to ovoid nuclei and small but visible nucleoli [Figure 1a–c]. Pseudopapillary aggregates lacking vessels in the center were present [Figure 2]. Cells ranged in appearance from round/oval epithelial to spindled [Figure 3]. Background contained metachromatic mucoid material staining magenta color with Diff Quik and pale blue on Papanicolaou stains [Figure 4]. Cell block contained epithelial cells arranged in loose sheets/aggregates and tubular/acinar pattern [Figure 5]. No significant atypia, mitosis, or necrosis was present.
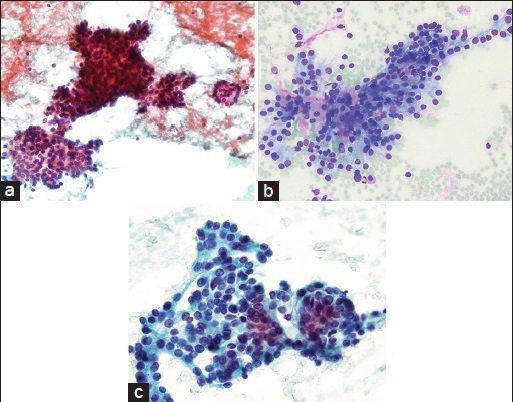
- (a) Cellular smear showing large branching and smaller acinar-like structures (Papanicolaou stain, ×20). (b) Branching architecture with magenta colored mucoid material surrounded by bland appearing cells with delicate cytoplasm (Diff Quik stain, ×20). (c) On this high power magnification, cells are uniform with nuclei that are round to ovoid in shape and small visible nucleoli. No necrosis or mitosis seen (Papanicolaou stain, ×40)
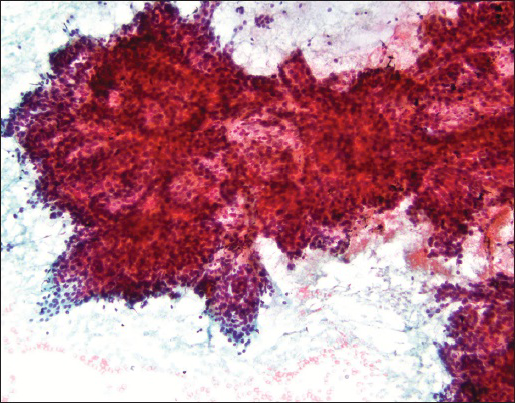
- A very large complex branching sheet with no visible fiborvascular cores (Papanicolaou stain, ×10)
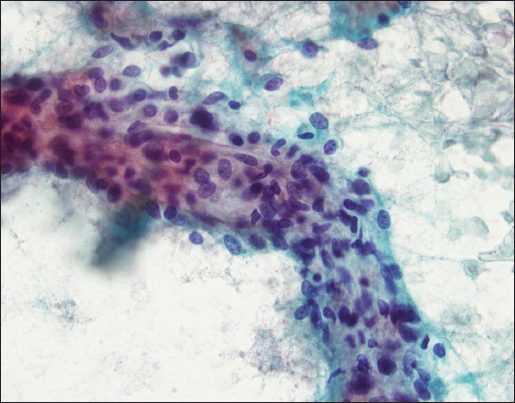
- A mixture of round, ovoid, and spindled cells are seen (Papanicolaou stain, ×40)
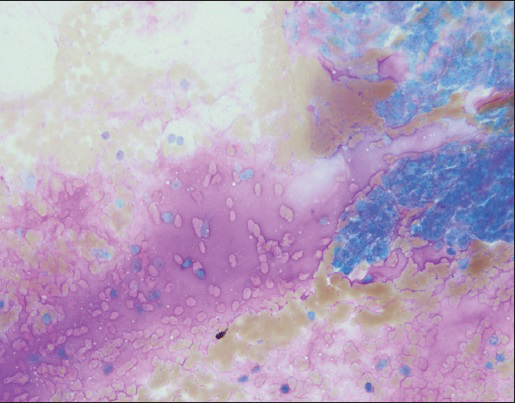
- Abundant magenta colored mucoid material is present in the background (Diff quik stain, ×20)
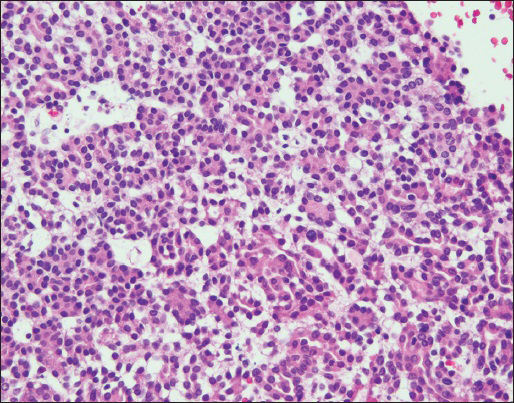
- Cell block shows back to back eosinophilic cells with acinar/tubular architecture. No mitosis, atypia, or necrosis seen (H and E stain, ×20)
Core biopsy revealed several small fragments of tumor cells with uniform low grade nuclei that formed a tubular/acinar architecture intermixed with mucinous material [Figure 6]. Immunohistochemistry studies showed that the tumor cells were positive for PAX8, CK7, and AMACR and negative for CD10.
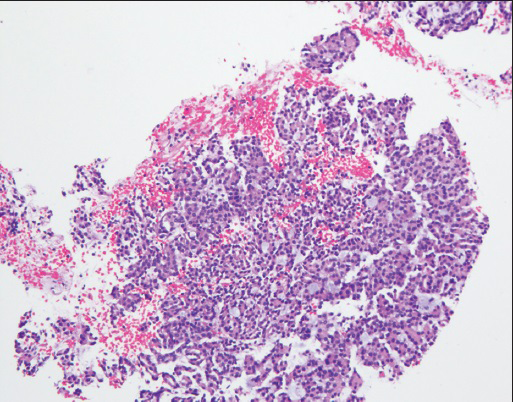
- Core biopsy: Findings similar to cell block- blue mucinous/mucoid material is seen within acinar/tubular structures (H and E stain, ×10)
A diagnosis of MTSCC was made.
Follow-up
A year later, the patient developed new and enlarging metastases to his liver from his colon carcinoma and was treated with ablation and embolization. The left renal MTSCC was not resected and continues to remain stable in size and appearance on imaging.
DISCUSSION
MTSCC is a clinically and morphologically distinct subtype of RCC.[2] Since its inclusion in the 2004 WHO classification of renal tumors, there has been isolated case reports in the literature of this entity. Recently, the largest single-institution series including 19 patients were published by Kenney et al.[1] In their series, the median age at diagnosis was 59 with a range of 17–71. Seventy-nine percent (79%) of cases were seen in females in contrast to other types of RCC which show male predominance. Most patients were treated with partial or total nephrectomy. Only one patient had active surveillance. Two patients developed metastasis with only one succumbing to disease. All others were free of recurrence or progression (median follow-up 40.1 months). Thus authors concluded that MTSCC is a renal tumor with an excellent prognosis but it is not universally indolent.
To the best of our knowledge, MTSCC was first described by Ordóñez and Mackay in 1996 as “RCC with unusual differentiation.”[41] Until 2004 WHO classification of renal tumors classified these tumor as MTSCC, they were reported under different names, and likely many were signed out by pathologists as RCC or its better known variants.
Macroscopically MTSCCs are solid well-circumscribed tumors with tan yellow cut surface with or without foci of necrosis or hemorrhage.[5] Histologic features most resemble type 1 papillary RCC. MTSCC lacks true papilla formation. MTSCC is histologically characterized by spindled and low cuboidal cells arranged in elongated tubules, cords, and trabecular arrangements.[678] Tumor cells have scant eosinophilic or clear/vacuolated cytoplasm and uniform nuclei. The cells are embedded in a mucinous or myxoid stroma. Spindled cells are bland appearing and vary in amount with some tumors having abundant spindled cells others having very few.[1] Immunohistochemically, the tumors are positive for AMACR, epithelial membrane antigen, CK7, and vimentin. CD-10 and RCC-Ma are often negative. The origin of this tumor remains unclear; distal tubule origin is favored based on immunohistochemical staining pattern. It is important to emphasize that some MTSCCs may be “mucin-poor” and show a marked predominance of elongated tubules lined by cuboidal epithelial cells or cords of spindled cells.[9] Fine et al. stated that this mucin-poor pattern coupled with the presence of other unusual features such as clear cells, papillations, foamy macrophages, and necrosis, may mimic other forms of RCC, particularly the papillary type. Pathologists must be aware of the spectrum of histologic findings within the MTSCCs to ensure their accurate diagnosis.
Molecular and chromosomal studies reported to date in MTSCC also overlap with papillary RCC. A review of the literature by Zhang et al. indicated that MTSCC is characterized by a variety of chromosomal alterations, including frequently losses of chromosomes 1, 4, 6, 8, 9, 11, 13, 14, 15, 22, and Y and gains of chromosomes 2, 5, 7, 9, 10, 11, 12, 16, 17, 18, 19, 20, 21, 22, and X.[10] Authors concluded that the MTSCC may be a subtype of papillary RCC based on morphologic, immunohistochemical, and chromosomal similarities.
To the best of our knowledge, there are only 5 case reports with a total of 6 patients in the literature describing the cytologic features of MTSCC on FNA.[37111213] All of these cases were diagnosed as RCC on FNA. Subsequent excisions showed MTSCC. Described cytologic features were identical to our case and can be summarized as follows.
-
Cellular smears
-
Cohesive branching sheets, psudopapillary clusters, and broad trabecular arrangements
-
Epithelial and spindled cells in varying amounts
-
Low grade nuclei-nuclei ranging from round, ovoid to spindled-visible but small nucleoli
-
Eosinophilic delicate and sometimes vacuolated or bubbly cytoplasm
-
Myxoid/mucinous background-metachromatic, magenta colored on Diff Quik and pale blue/green on Papanicolaou stains
-
No necrosis, hemorrhage, and cystic change.
Tubule and cord formation so prominently seen on histologic sections is not a predominant feature in cytologic specimens. If there is a cell block, such was in our case; tubule formation is more readily seen.
Differential diagnosis on cytology includes conventional clear cell RCC and papillary RCC. Cytologically clear cell RCC usually shows a predominance of clear epithelial cells arranged in loosely cohesive groups with single cell in the background. Nuclei are round with variable atypia-grade 1 nuclei showing no visible nucleoli and higher grade tumors showing more significant atypia and prominent nucleoli. Epithelial cells have abundant fragile cytoplasm, usually pale eosinophilic, vacuolated, or granular. Clear cell morphology is usually not well represented in cytologic preparations.[14] Necrosis, hemorrhage, and cystic change are common in the background. In papillary RCC, cells aggregate around fibrovascular cores, in addition to dispersed cells. In type 1, cells are pale with low grade nuclei, some with grooves. In type 2, cells are large and eosinophilic with prominent nucleoli. Background contains blood, necrotic debris, foamy macrophages, and hemosiderin pigment.[14] Neither clear cell RCC or papillary RCC contain bland spindled cells or mucin/mucoid matrix common to MTSCC. On the other hand, necrosis and cystic change common to clear cell and papillary RCC are not encountered in the MTSCC. Immunohistochemical stains on cell block preparations are helpful to differentiate MTSCC from conventional clear cell RCC but not papillary RCC. AMCAR and CK7 positivity in MTSCC is similar to papillary RCC.[1] MTSCC is usually negative for CD10 and RRC marker which are common in conventional RCC.
Most renal tumors are not sampled by FNA prior to surgical excision. Utility of renal FNA is mostly to rule out a metastasis to the kidney when there is a new diagnosis or a known history of another primary malignancy in the setting of a renal mass. Given the rarity of this tumor combined with a very few cases reported in the cytology literature, it is likely that most pathologists are unfamiliar with this entity on FNA cytology. This report summarizes the cytologic features in detail. We believe that once the pathologist is aware of this tumor in the differential diagnosis, its accurate diagnosis is possible on FNA due its distinctive features; mainly a low grade tumor with epithelial and spindled cells in a myxoid background.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
No competing interest to declare by any of the authors.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author acknowledges that this final version was read and approved. All authors qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article
ETHICS STATEMENT BY ALL AUTHORS
All authors abide by the institutional ethical guidelines.
LIST OF ABBREVIATIONS (In alphabetic order)
CT - Computed Tomography
FNA - Fine Needle Aspiration
MTSCC - Mucinous Tubular and Spindle Cell carcinoma
RCC - Renal Cell Carcinoma
WHO - World Health Organization.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2015/12/1/28/171135
REFERENCES
- Mucinous tubular and spindle cell carcinoma (MTSCC) of the kidney: A detailed study of radiological, pathological and clinical outcomes. BJU Int. 2015;116:85-92.
- [Google Scholar]
- 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798-805.
- [Google Scholar]
- Fine needle aspiration biopsy of renal mucinous tubular and spindle cell carcinoma: Report of two cases. Diagn Cytopathol. 2010;38:51-5.
- [Google Scholar]
- Renal cell carcinoma with unusual differentiation. Ultrastruct Pathol. 1996;20:27-30.
- [Google Scholar]
- Renal mucinous tubular and spindle cell carcinoma: A report of 8 cases and review of the literature. Diagn Pathol. 2013;8:206.
- [Google Scholar]
- The imaging and pathological features of a mucinous tubular and spindle cell carcinoma of the kidney: A case report. World J Surg Oncol. 2013;11:34.
- [Google Scholar]
- Mucinous tubular and spindle cell carcinoma of the kidney: A case report. Case Rep Oncol. 2012;5:347-53.
- [Google Scholar]
- Expanding the histologic spectrum of mucinous tubular and spindle cell carcinoma of the kidney. Am J Surg Pathol. 2006;30:1554-60.
- [Google Scholar]
- Mucinous tubular and spindle cell carcinoma and solid variant papillary renal cell carcinoma: A clinicopathologic comparative analysis of four cases with similar molecular genetics datum. Diagn Pathol. 2014;9:194.
- [Google Scholar]
- Mucinous tubular and spindle cell carcinoma of the kidney: Cytopathologic findings. Diagn Cytopathol. 2007;35:593-6.
- [Google Scholar]
- Cytologic aspect of mucinous tubular and spindle-cell renal carcinoma in fine-needle aspirates. Diagn Cytopathol. 2006;34:660-2.
- [Google Scholar]
- Fine needle aspiration cytology of a low grade myxoid renal epithelial neoplasm: A case report. Acta Cytol. 2005;49:525-9.
- [Google Scholar]
- Kidney, adrenal and retroperitoneum proper. In: Orell and Sterrett's Fine Needle Aspiration Cytology. Elsevier Limited; 2012. p. :316-38.
- [Google Scholar]








