Translate this page into:
Cytopathology of neoplastic meningitis: A series of 66 cases from a tertiary care center
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Neoplastic meningitis (NM) is a condition characterized by leptomeningeal involvement by metastatic carcinoma. Detection of exfoliated malignant cells in cerebrospinal fluid (CSF) due to meningeal metastasis is frequently associated with diverse neurologic presentations.
Materials and Methods:
In this retrospective study of all cases of NM diagnosed in CSF samples over a 20-year period at a tertiary care referral center, the cytomorphologic features were reviewed.
Results:
Sixty six cases of NM were identified of which 36 already had an established diagnosis of malignancy while in 30 patients, there was no previously known tumor. The most common known primary in the former group was breast followed by ovary. Single cell pattern, cellular cannibalism, moderate cytoplasm and rounded nuclei were seen in breast and lung tumors. Papillary architecture and cytoplasmic vacuolation were seen in the ovarian primaries. Melanin pigment was seen in malignant melanoma.
Conclusion:
CSF cytology is an important tool for diagnosis of NM. Cytomorphologic features helped in diagnosis and for prediction of the primary site. Correct identification of this condition is important as it has therapeutic and prognostic implications.
Keywords
Cerebrospinal fluid
cytology
neoplastic meningitis
non-lymphoreticular malignancy
pathology
INTRODUCTION
Cytologic examination of cerebrospinal fluid (CSF) is routinely performed during the management of patients with malignancies that frequently have leptomeningeal spread, including patients with acute lymphoid leukemias (ALL) and medulloblastomas. Cytology laboratories in large centers treating cancer patients occasionally come across meningeal carcinomatosis or neoplastic meningitis (NM), in which there is diffuse seeding of the CSF by metastatic carcinoma cells.[1] Detection of exfoliated malignant cells in CSF due to the meningeal carcinomatosis may sometimes be the first diagnostic clue in patients with diverse neurological symptoms. While CSF cytology is highly specific (>95%) in detection of cancer cells, it suffers from a lack of sensitivity (<50%).[2] In addition, the detection of metastatic carcinoma cells in CSF of patients suffering from an extracranial carcinoma also has important therapeutic and prognostic implications. NM is a clinically important condition and numerous reports on cytology are available in literature; however, mostly from early 80s and late 90s.[345678]
The central nervous system (CNS) is becoming a more common site of involvement as cancer patients live longer due to improvements in systemic therapies and advancement in neuroimaging studies. Moreover, many chemotherapeutic agents do not cross the blood brain barrier, leaving the CNS/meninges as a potential tumor hideout.
This is increasing the importance of effective detection methods of cancer cells in the CSF as early management can increase the therapeutic benefit. There are three methods to detect NM, which include clinical signs and symptoms, magnetic resonance imaging (MRI) of the brain and spine and CSF cytology. Clinical manifestations are often non-specific. MRI sensitivity and specificity vary with the type of primary cancer with a detection rate of NM in fewer than 50% of patients. On the other hand, CSF cytology has a high specificity (>95%) but associated with less sensitivity.[23]
This retrospective study was undertaken to evaluate the utility of CSF cytopathology for the diagnosis of metastatic malignancies and to describe cytomorphologic features of various tumors, which help in diagnosis of NM.
MATERIALS AND METHODS
This study was a retrospective analysis of all CSF samples, obtained by spinal tap exclusively and received in the cytopathology laboratory over a period of 20 years from January 1993 to June 2012. Some patients had more than one sample of CSF as part of diagnostic analysis. In most cases, CSF samples were processed by cytocentrifugation technique (Cytospin®) and stained by Papanicolaou and May Grunwald Giemsa (MGG) stains. In nine cases, filter membrane preparations (Millipore®) were available. Immunostaining with cytokeratin (CK) (Thermo Scientific, CA, USA) and epithelial membrane antigen (EMA) (Thermo Scientific, CA, USA) was carried out in four cases.
All cases reported positive and suspicious for metastatic carcinoma cells were included in the study. Clinically, these patients presented with neurologic deficits and thus were subjected to CSF cytologic examination. Imaging details such as MRI features were not available due to the retrospective nature of the study. Slides were reviewed by four Cytopathologists (GS, SRM, VKI, DJ) and classified based on the consensus into negative, positive and suspicious categories; when multiple CSF samples had been examined for a patient, the highest category of cytologic classification was selected.
RESULTS
A total of 15,430 CSF samples were evaluated during the study period of 20 years. Three hundred thirty five of these specimens from 292 patients were positive or suspicious for malignant non-lymphoreticular tumors, whereas 755 specimens from 548 patients were positive or suspicious for lymphoreticular tumors [Tables 1 and 2].
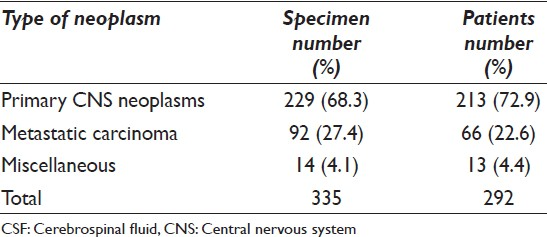
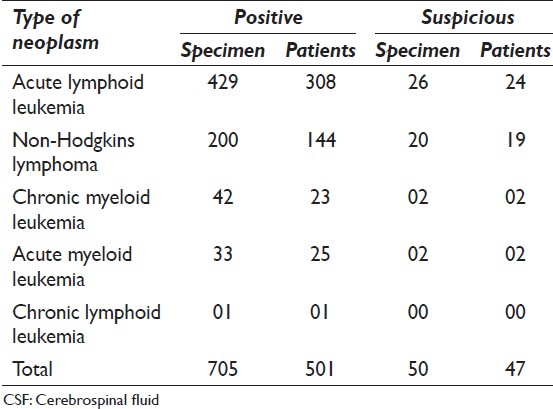
The most common non-lymphoreticular tumors were primary CNS neoplasms (72.9%) followed by metastatic carcinomas (22.6%). The most common lymphoreticular tumors were ALL (60.2%) followed by non-Hodgkins lymphomas (29.1%).
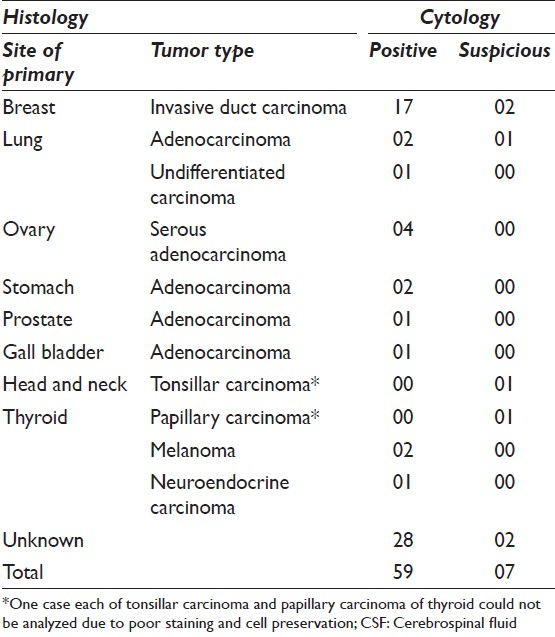
Ninety two specimens from 66 patients had a diagnosis of metastatic carcinoma. 36 (54.6%) patients had already been diagnosed with systemic malignancy whereas 45.4% of the cases presented with neurological symptoms. In the former, the most common primary was breast (28.7%) followed by ovary (6%) and lung (4.5%) [Table 3].
The age of patients with NM ranged from 22 years to 79 years with a mean of 50.5 years. There were 22 males and 44 females (M:F ratio of 1:2).
Breast carcinoma (19 cases)
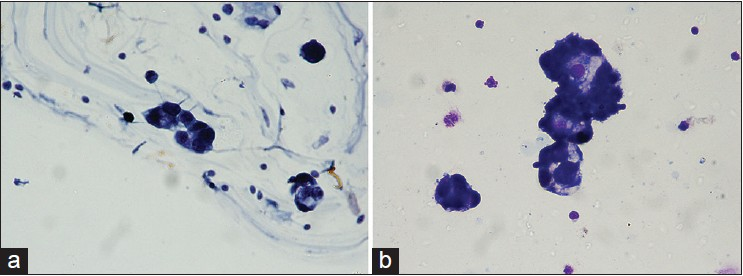
CSF smears of patients with metastasis from breast carcinoma were highly cellular [Figure 1a]. The tumor cells were arranged mainly singly [Figure 1b], although an occasional small cohesive cluster of tumor cells could be seen in most samples. Binucleate and multinucleate cells were also seen [Figure 1c]. A cell in cell appearance with one cancer cell engulfing an adjacent cancer cell (cellular cannibalism) was seen in most of the cases [Figure 1d]. The cells contained central nuclei with a moderate amount of dense cytoplasm with only occasional cell showing fine peripheral vacuolation [Figure 1e]. Prominent eosinophilic nucleoli most often multiple were seen in all cases [Figure 1f].

- Cerebrospinal fluid cytospin preparation from a case of carcinoma breast shows highly cellular smear (a) with largely dyscohesive atypical cells (b); frequent multinucleation (c) and cellular cannibalism is evident (d). Individual cells show fine cytoplasmic vacuolations (e) and prominent nucleoli (f). (a: May GrunwaldGiemsa, ×200, b: Pap, ×400, c-f: Pap, ×400)
Ovarian carcinoma (four cases)
The cellularity of cytospin CSF preparation was high with an abundance of cell clusters, which in the majority of cases exhibited papillary pattern [Figure 2a]. Cytoplasmic vacuolation was a conspicuous feature [Figure 2b].
- Case of carcinoma ovary shows tight papillary clusters (a: Pap, ×400) and cytoplasmic vacuolation (b: May GrunwaldGiemsa, ×400)
Melanoma (two cases)
Singly lying cells with high nuclear cytoplasmic ratio and hyperchromatic nuclei were seen [Figure 3a]. Intracytoplasmic coarse black granular pigment was identified in a significant number of tumor cells as well as within histiocytes, which failed to stain with iron (Prussian blue) stain [Figure 3b] and positive for melanin bleach stain.

- Cytospin preparation of CSF of a case of melanoma displays large atypical cell with high nuclear cytoplasmic ratio present within hemorrhagic background; coarse black pigment is seen in the cytoplasm of an adjacent degenerated cell (a: May GrunwaldGiemsa, ×400). The pigment fails to stain for Prussian blue stain for iron (b). Increase cellularity of singly lying atypical cells is observed in majority of cases of unknown primary site of cancer (c: Pap, ×400)
Lung (4), stomach (2), gallbladder (1), prostate (1) and neurondocrine carcinoma (1): No specific features were identified due to a small number of cases. The tumor cells mainly showed a dyscohesive pattern with singly lying cells having moderate cytoplasm.
One case each of tonsillar carcinoma and papillary carcinoma of thyroid could not be analyzed due to poor staining and cellular preservation.
Unknown primary (30 cases)
Overall cellularity in the majority of these cases was scanty to moderate. The tumor cells were present singly in a majority (25) of the cases [Figure 3c]. The cells showed moderate to abundant cytoplasm with cytoplasmic protrusions. No mucin or melanin pigment was identified. The site of primary was suggested based on subtle morphologic features; however, no follow-up could be obtained and exact primary remained unknown.
In general, three patterns of CSF involvement were recognized.
Pattern I consisted of a large number of single cells predominating on CSF cytology. The tumor cells had moderate cytoplasm and centrally placed nuclei showing mild to moderate nuclear pleomorphism. This pattern was largely seen in metastatic breast and lung carcinoma [Figure 4a].

- A case of adenocarcinoma of lung shows singly lying cells with pleomorphic nuclei (a: Pap, ×400). Milipore membrane filtration preparation shows a cluster of atypical cells from another case of pulmonary adenocarcinoma (b: Pap, ×400). Immunocytochemistry for cytokeartin shows strong cytoplasmic positivity in a case of ovarian carcinoma (c)
Pattern II consisted of large cells with abundant cytoplasm with frequent cell groupings and clusters. Marked cytoplasmic vacuolation was a frequent finding in this group [Figure 2b]. Ovarian carcinoma exhibited this pattern.
Pattern III was characterized by the presence of necrotic debris, inflammatory background with a few tumor fragments. Few lung carcinomas, melanomas and cases of unknown primary showed pattern III [Figure 4b].
Immunocytochemistry (ICC) for CK and EMA was performed in 4 cases of breast (2) and ovarian (2) carcinoma. All cases showed strong cytoplasmic positivity [Figure 4c]. In other cases, ICC could not be carried out due to either scanty cellularity or a limited number of diagnostic slides. Further ICC for diagnosis of primary site could also not be attempted in any of the cases as the quantity of CSF sample obtained was always limited.
DISCUSSION
Leptomeningeal metastasis is a serious complication of cancer that occurs in 5-10% of patients with solid tumors and is observed most commonly in patients with breast cancer, lung cancer or melanoma.[91011] Nearly, 20% of patients with neurological symptoms and signs are found to have meningeal carcinomatosis at autopsy.[10]
Tumor cells reach the leptomeninges by hematogenous spread or by direct extension from lesions and are then disseminated throughout the nervous system by the flow of the CSF.[1] Unfortunately, false negative results are common. It has been suggested that false negative results can be minimized by withdrawing more CSF for cytologic analysis, processing the CSF specimen immediately and repeating the procedure if the initial cytology is negative.[5]
Although there are ancillary markers or techniques available, which are helpful in detection of CSF dissemination such as CSF levels of protein, glucose and tumor markers or contrast-enhanced MRI, none of these have shown a definite increase in the diagnostic yield compared to the cytological examination.[6121314] Neuroimaging may establish or support the diagnosis in some patients. Radiographic abnormalities include leptomeningeal, subependymal, dural or cranial nerve enhancement. MRI plays an important confirmatory role and can demonstrate involved sites, even in cytology negative cases.[15] However, almost similar character of enhancement in the presence of infective, inflammatory or neoplastic process is the reason for the restricted specificity of MRI. On comparative analysis, MRI is found to have equal sensitivity to CSF cytology, but suffers from lesser specificity.[16]
Cytopreparatory and staining techniques are also important in accurate evaluation of CSF specimens. Several reports in the literature deal with a comparison of various techniques.[1718192021] In our experience, smears made by Cytospin® cytocentrifuge technique and one slide each stained by Papanicolaou and MGG technique gives the maximum information. Filter membrane preparation may be reserved for cases with low yield or diagnostic problems. In our laboratory, we use filter membrane preparation for the diagnosis of primary CNS neoplasms where low yield is expected; however, this method suffers from the drawbacks of exhaustion of complete fluid; hence, further ancillary studies cannot be carried out. Thin monolayer technology has been suggested as an appropriate diagnostic method for metastatic tumors in CSF.[22]
A number of large series have described the statistical information regarding the prevalence of various primary carcinoma sites, which cause NM.[4678] Few studies have documented the morphologic peculiarities of carcinoma cells found in the CSF depending on the primary sites.[78] The diagnosis of meningeal involvement is relatively easy when the patient has a history of carcinoma. Patients with neurological signs and symptoms of NM without any known primary site are more difficult to diagnose on cytology unless there is a high index of suspicion.
This study presents the cytomorphologic findings of NM in 66 patients. Similar to several previous reports, the most common sites were breast, lung, stomach and melanoma.[91011] Most of the neoplasms were adenocarcinomas as reported by others.[232425] We did not come across any squamous cell carcinoma, which is considered a rare diagnosis in CSF cytology.[26]
CNS metastases occur in approximately 35% of patients with breast cancer, which is associated with poor survival[27] and only 50% of patients with leptomeningeal carcinomatosis are diagnosed.[13] Morphologic analysis of breast carcinoma patients was characterized by the presence of singly scattered isolated cells with minimal aggregation. This pattern was without characteristics of the original tumor architecture and signifies extensive involvement of the subarachnoid space. In contrast to earlier reports, characteristic Indian file pattern was not conspicuous in our cases.
Ovarian cancer does not commonly involve the nervous system. Leptomeningeal metastasis is an even rarer complication of ovarian cancer with only a handful cases reported in the literature.[28] We have identified four cases of ovarian carcinoma in the present series with characteristic cytomorphologic features. Ovarian malignancies showed largely grouping of cells with papillary architecture (pattern II) and prominent cytoplasmic vacuolation, which was degenerative in nature. Pattern III was characterized by the presence of necrotic material with a few large tumor fragments and the presence of an inflammatory background, which may represent intraparenchymal metastasis; however, it could not be correlated with imaging studies. We have identified the same pattern in lung carcinomas, melanomas and cases of unknown primary malignancies. There were 30 cases where positive CSF cytology was the first evidence of malignancy. Although, exact site of primary was not identified due to either lack of follow-up or early demise of the patient due to disseminated malignancy, report of positive cytology helped the clinician in the overall management and prognostication of patient.
Multidisciplinary therapy, such as radiation and chemotherapy can improve quality-of-life in patients of meningeal metastasis.
Role of ICC in diagnosis of NM is limited and is of minor help in the problem of false negative cytology. Combined use of conventional cytology and ICC leads to a slight increase of sensitivity in detecting malignant cells compared with cytology alone.[29]
An overview of research tools in the field of CSF cancer detection reveals a variety of technologies that can be used to understand the biology of metastatic cancer. Methods currently under investigation include new ICC methods and flow cytometry for detection of cells.[30] In addition, polymerase chain reaction, fluorescence in situ hybridization and mass spectrometry are being tested for assessment of the non-cellular biomarkers in CSF.[31]
In primary CNS neoplasms (ependymoma [7] retinoblastoma [117], medulloblastoma [86] and pineoblastomas [3]), the malignant cells appear in groups of cells, which seldom allow for the histological identification of the original tumor. Grouping of the cells may mimic cohesive cell clusters of meningeal carcinomatosis. However, age of the patient, known diagnosis, high nuclear cytoplasmic ratio and nuclear molding were helpful features to distinguish these from carcinoma cells. In these cases, the CSF cytology is useful only for confirmation of the presence of malignant cells and has no role in histological definition.[4] Leukemia and lymphoma cells retain original characteristics of the tumor and the severity of the disease is not reflected necessarily in the CSF cellularity, which may vary from scanty to elevated.[4] A positive CSF cytology result, especially in cases of metastatic non-lymphoreticular neoplasms is highly reliable and false positive diagnoses are rare in experienced hands.
Therefore, an attempt must be made to differentiate primary CNS neoplasms from metastatic tumors on the basis of cytomorphology of tumor cells. Identification of the type of metastatic neoplasm facilitates the detection of the site of the primary neoplasm in such cases.
CONCLUSION
This study presents cases of NM diagnosed over a period of 20 years at a tertiary care referral center; however, limited by follow-up information of cases, in which primary site of malignancy remained unknown. Cytologic examination of CSF is an important modality to diagnose meningeal involvement by the systemic malignancies. There are cytomorphologic clues by which this condition is diagnosed and site of primary cancer may be identified.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
GS participated in analysis of data and drafting of manuscript. SRM and VKI contributed in the design of study, acquisition of data, analysis and interpretation of data. DJ has been involved in drafting the manuscript, revising it critically for important intellectual content, analysis and interpretation of data.
ETHICS STATEMENT BY ALL AUTHORS
All authors take the responsibility of maintaining relevant documentation of records, slides and other data of cases used in this study on archival material as per the Institutional policy.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind mode (authors are blinded for reviewers and vice versa) through automatic online system
Available FREE in open access from: http://www.cytojournal.com/text.asp?2013/10/1/13/114212
REFERENCES
- Diagnostic tools for neoplastic meningitis: Detecting disease, identifying patient risk, and determining benefit of treatment. Semin Oncol. 2009;36:S35-45.
- [Google Scholar]
- Cerebrospinal fluid (CSF) cytology: Current status and diagnostic applications. J Neuropathol Exp Neurol. 1992;51:235-45.
- [Google Scholar]
- Cerebrospinal fluid cytology: An 11-year experience with 5951 specimens. Arch Pathol Lab Med. 1998;122:47-51.
- [Google Scholar]
- Cerebrospinal fluid cytology in patients with cancer: Minimizing false-negative results. Cancer. 1998;82:733-9.
- [Google Scholar]
- Cerebrospinal fluid cytology and clinical analysis of 34 cases with leptomeningeal carcinomatosis. J Int Med Res. 2009;37:1913-20.
- [Google Scholar]
- Cytopathology of nonlymphoreticular neoplasms metastatic to the central nervous system. Acta Cytol. 1981;25:599-610.
- [Google Scholar]
- The cytopathology of cerebrospinal fluid. II. Metastatic cancer, meningeal carcinomatosis and primary central nervous system neoplasms. Acta Cytol. 1981;25:461-79.
- [Google Scholar]
- Diagnostic value of cerebrospinal fluid level of carcinoembryonic antigen in patients with leptomeningeal carcinomatous metastasis. J Clin Neurol. 2010;6:33-7.
- [Google Scholar]
- Diagnostic value of cerebrospinal fluid cytology in comparison with tumor marker activity in central nervous system metastases secondary to breast cancer. Cancer. 1993;72:2376-82.
- [Google Scholar]
- Leptomeningeal metastases in pediatrics: Magnetic resonance image manifestations and correlation with cerebral spinal fluid cytology. Pediatr Int. 2010;52:541-6.
- [Google Scholar]
- Neuroradiologic findings in leptomeningeal carcinomatosis: The value interest of gadolinium-enhanced MRI. Neuroradiology. 1990;32:26-32.
- [Google Scholar]
- The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol. 1999;246:810-4.
- [Google Scholar]
- Simple method for the cytological examination of cerebrospinal fluid. J Clin Pathol. 1977;30:486-7.
- [Google Scholar]
- Cytology of cerebrospinal fluid in the diagnosis of malignancy. J Neurosurg. 1968;28:317-26.
- [Google Scholar]
- Diagnostic cytology of cerebrospinal fluid by the cytocentrifuge method. Am J Clin Pathol. 1979;72:931-43.
- [Google Scholar]
- Cerebrospinal fluid cytology: Diagnostic accuracy and comparison of different techniques. Acta Cytol. 1976;20:542-7.
- [Google Scholar]
- Diagnosis of metastatic tumors in cerebrospinal fluid samples using thin-layer cytology. Acta Cytol. 2008;52:304-8.
- [Google Scholar]
- Cytologic evaluation of cerebrospinal fluid with clinical and histologic correlation. Acta Cytol. 1980;24:401-2.
- [Google Scholar]
- Cytodiagnosis of malignant lesions in cerebrospinal fluid.Review and cytohistologic correlation. Acta Cytol. 1985;29:286-90.
- [Google Scholar]
- Metastatic squamous cell carcinoma in cerebrospinal fluid: Why a rare diagnosis on cytology? Acta Cytol. 2012;56:209-13.
- [Google Scholar]
- Contribution of CSF cytology in the diagnostic work-up of breast cancer patients with neurological symptoms: A retrospective analysis over two decades. J Neurooncol. 2012;107:581-9.
- [Google Scholar]
- Case report and review of literature: Leptomeningeal relapse in epithelial ovarian cancer. Gynecol Oncol. 1994;54:227-31.
- [Google Scholar]
- CSF cytology versus immunocytochemistry in meningeal carcinomatosis. J Neurol Neurosurg Psychiatry. 1988;51:142-5.
- [Google Scholar]
- Flow cytometric immunophenotyping of cerebrospinal fluid specimens. Am J Clin Pathol. 2011;135:22-34.
- [Google Scholar]
- Detection of cancer cells in the cerebrospinal fluid: Current methods and future directions. Fluids Barriers CNS. 2011;8:14.
- [Google Scholar]








