Translate this page into:
Use of the term atypical cells in the reporting of ascitic fluid cytology: A caveat
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Abdominal paracentesis is a routine diagnostic procedure for assessment of patients with recent onset or worsening of ascites.
Objectives:
The objective of the study is to (1) review clinically confirmed cases of malignancy with negative, atypical, and suspicious cytology reports and provide reasoning for discrepancies and (2) recalculate sensitivity, specificity, and predictive values after review.
Materials and Methods:
Papanicolaou smears of ascitic fluid paracentesis samples received over one calendar year were reviewed retrospectively by an expert in cytopathology blinded to the final clinical and/or histopathological diagnoses. Cases with discrepancies after review were noted. Sensitivity, specificity, and predictive values were calculated before and after review of slides. Data were analyzed using SPSS version 16.
Results:
Malignant etiology was identified in 49/115 cases (42.6%) with female genital tract being the most common site of malignancy (22, 44.8%). The remaining 66 (57.4%) had a benign etiology with hepatic cirrhosis in 42 cases (63.6%). A review revealed discrepancies in five cases, three of which were earlier called negative for malignant cells (one case each of ovarian adenocarcinoma, cecal adenocarcinoma, and cholangiocarcinoma). Two cases of ovarian adenocarcinoma that were reported as atypical/reactive mesothelial hyperplasia showed malignant cells upon review. Sensitivity and specificity after review were 69.4% and 100%, respectively, with 100% positive predictive value.
Conclusion:
Being a minimally invasive procedure, abdominal paracentesis continues to be an important diagnostic tool in guiding patient management. A proper morphological assessment with adequate clinical information and correlation with other investigations can be used to arrive at a definitive diagnosis in most cases. The term “atypical” can be misleading and is often used for want of clinical information and is best avoided.
Keywords
Atypical cytology
atypical mesothelial cell
cytology smear
effusion cytology
reactive mesothelial cells
INTRODUCTION
Diagnostic abdominal paracentesis is the recommended procedure for assessment of a patient with either a recent onset or worsening of ascites. Decompensated hepatic cirrhosis is the leading cause worldwide, and the ascitic fluid is usually transudate in nature with a serum-ascites albumin gradient (SAAG) >1.1 g/dl.[12345] Ascites is the first sign of malignancy in around half of all patients with peritoneal carcinomatosis secondary to malignancies of the gynecological and gastrointestinal tracts.[6789] Detection of malignant cells on effusion cytology in these patients is important for management and disease prognostication.[101112] Both architecturally and cytologically reactive mesothelial cells may pose a diagnostic challenge. The reported false-negative rate of ascitic fluid smear cytology for malignancy is as high as 42%. The sensitivity and specificity range between 22% and 81% and 97% and 100%, respectively.[13] The aim of our study was to review cytology smears of clinically confirmed malignant cases with negative, atypical, and suspicious cytology reports with a reasoning for discrepancies before and after review. Sensitivity, specificity, and predictive values were recalculated.
MATERIALS AND METHODS
Patients who underwent cytological evaluation of ascitic fluid over a calendar year (January–December 2012) at constituent teaching hospitals of our institution were identified retrospectively. Clinical details including patient history, physical examination, laboratory findings, and radiology and histopathology reports were retrieved from patient medical records. A histopathology report was used to confirm the diagnosis of malignancy, and when this was not available, a diagnosis made by correlating fine-needle aspiration cytology (FNAC), serum tumor markers, and imaging studies chiefly ultrasonography and computed tomography scan was considered. SAAG was used to classify ascitic fluid as transudate (gradient >1.1 g/dL) or exudate (≤1.1 g/dL). The study was approved by the Institutional Ethics Committee.
Smears from ascitic fluid were prepared by centrifuging 10 ml of fluid at 1500 rpm for 5 min. The sediment obtained after discarding the supernatant was smeared on slides, immediately fixed in alcohol, and stained with Papanicolaou (Pap) stain. The smears were being reported as positive, negative, atypical, and suspicious with regard to the presence of malignant cells. Smears were re-examined by an expert in cytopathology (author 1) who was blinded to the final diagnosis.
At the time of review of slides under low power, cellularity of the smear and presence of two cell populations were considered while reporting. Single cells that exhibited atypia in the form of high nuclear/cytoplasmic ratio, nuclear enlargement and hyperchromasia, irregular nuclear membrane contour, coarsely clumped chromatin, and prominent nucleoli were considered malignant. Atypical/reactive mesothelial cells were characterized by a peripheral light ectoplasm and an inner dark endoplasm. The nucleus was uniform, central to eccentrically placed, but not touching the cell border with a micro- to macronucleolus. Architecturally complex mesothelial cells arranged in a papillary-like configuration with knobby borders predominantly formed by cytoplasm, peripheral ruffled borders, and windowing were considered characteristics of reactive cells occurring in groups.
Cytology smears reported as suspicious for malignancy were grouped along with positive cases, and those that were reported as atypical/reactive were grouped along with negative cases for calculation of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). These values were revised after review. SPSS for Windows (version 16.0; SPSS Inc., Chicago, Illinois, USA) was used for statistical analysis. For categorical data, proportions were calculated and Chi-square was applied for noncategorical data with P < 0.05 as statistically significant. For continuous data, mean was used and the values were expressed as 95% confidence interval. The utility of ascitic fluid cytology smear as a diagnostic tool for malignancy was calculated using respective formulae.
RESULTS
We identified a total of 115 patients who had undergone diagnostic paracentesis for varied indications. The median age of the population was 56.5 years with a male-to-female ratio of 1:1.16. A malignant etiology was confirmed in 49 (42.9%) cases by histopathology in 31 and by other means such as radiological findings, elevated serum tumor marker levels, and FNAC results in the remaining 18. A nonmalignant etiology was identified in 66 (57.4%) cases with decompensated hepatic cirrhosis being the most common cause in 42 cases. Majority of these patients presented with moderate (79 cases, 69.3%) to massive (19 cases, 16.7%) ascites with associated pleural effusions in 27 cases (23.68%). A breakdown of final diagnoses against cytological results before and after review of slides by the expert is presented in Table 1.
| Clinical diagnosis | Ascitic fluid cytology | |||||||
|---|---|---|---|---|---|---|---|---|
| Before review | After review | |||||||
| Positive | Suspicious | Atypical | Negative | Positive | Suspicious | Atypical | Negative | |
| Malignancy | ||||||||
| FGT and breast | 12 | - | 3 | 5 | 15 | - | 1 | 4 |
| Gastrointestinal | 9 | 1 | - | 3 | 10 | 1 | - | 2 |
| Pancreaticobiliary | 5 | - | - | 3 | 6 | - | - | 2 |
| Unknown primary | 2 | - | - | 1 | 2 | - | - | 1 |
| Hepatocellular carcinoma | - | - | - | 4 | - | - | - | 4 |
| Lymphoma | - | - | - | 1 | - | - | - | 1 |
| Total (n=49) | 28 | 1 | 3 | 17 | 33 | 1 | 1 | 14 |
| Other diagnoses | ||||||||
| Cirrhosis | - | - | 1 | 41 | - | - | 1 | 41 |
| Abdominal tuberculosis | - | - | 1 | 7 | - | - | 1 | 7 |
| Serious infection | - | - | - | 5 | - | - | - | 4 |
| Benign disease of ovary | - | - | - | 6 | - | - | - | 6 |
| Miscellaneous | - | - | - | 5 | - | - | - | 5 |
| Total (n=66) | 0 | 0 | 2 | 64 | 0 | 0 | 2 | 64 |
FGT: Female genital tract
On review, there were 20 false-negative cases on cytology which included malignancies of the female genital tract (FGT) and breast (8), hepatocellular carcinoma (HCC) (4), gastrointestinal tract malignancy (3), pancreaticobiliary malignancy (3), and one case each of Non-Hodgkin's lymphoma and omental metastasis from an unknown primary. The review revealed discrepancies in five cases, three of which were earlier called negative for malignant cells (one case each of ovarian adenocarcinoma, cecal adenocarcinoma, and cholangiocarcinoma). Two cases of ovarian adenocarcinoma that were reported as atypical/reactive mesothelial hyperplasia showed malignant cells on review.
The sensitivity and specificity of conventional smear cytology in the detection of malignancy before review were 59.2% and 100%, respectively. PPV was 100% and NPV was 76.7%. Values after review were as follows: sensitivity, 69.4%; specificity, 100%, PPV, 100%; and NPV, 81.5%. Other laboratory parameters including SAAG, cell count, and ascitic fluid biochemistry showed little difference between the two groups. The mean SAAG of the malignant and nonmalignant groups was 1.36 ± 0.14 and 1.76 ± 0.10, respectively, with P = 0.275. The mean SAAG of cytological true positives and false negatives was 1.13 ± 0.18 and 1.67 ± 0.22, respectively (P = 0.579).
DISCUSSION
The underlying pathophysiological mechanism of ascitic fluid formation determines the number and type of cells found on cytological examination of the fluid.[1314] In our study, nonmalignant etiologies constituted the larger group and were similar to findings in other studies with hepatic cirrhosis being the single most common etiology.[6789] Cytological examination of ascitic fluid from all 42 patients was negative for malignant cells. Karoo et al. in their study argue that smear cytology should be performed in patients only when there is a strong clinical suspicion of malignancy. They reported a total of 184 cases (67%) where an unnecessary diagnostic paracentesis was performed due to the lack of a hospital protocol.[9] The presence of malignant cells on ascitic fluid smear cytology may be the first indication of malignancy in around half of all patients with peritoneal carcinomatosis and is generally regarded as a poor prognostic sign.[10111215] Malignancies of the FGT, primarily from the ovary in 12 cases, and peritoneal carcinomatosis (9) with primaries in the stomach, colon, pancreas, and gallbladder were positive for malignant cells on cytology in our study. Other studies show a similar distribution of cases.[6789]
A high false-negative rate is a well-recognized problem with conventional smear cytology with reported rates being as high as 42%. The false-negative rate in our study was 40.82%. There are mechanisms by which a malignant tumor can cause ascites but still be negative on cytology.[12] All four confirmed cases of HCC in our study manifested with ascites. The fluid from these patients was transudate in nature and cytologically negative for malignant cells. The cause for ascites in these cases was likely due to the underlying cirrhosis, in reality making them cytologically true negative. Thrall et al. in their study state that peritoneal involvement in HCC is rare and may not reveal malignant cells on fluid cytology even in the presence of a strong clinical and/or radiological suspicion. A positive smear is these cases is usually suggestive of peritoneal invasion.[1617] Motherby et al. attribute the high false-negative rate in their study to three factors, namely screening error by the cytologist, sampling error by the physician, or a cause for effusion unrelated to the malignancy.[13] In our study, three cases that were initially reported negative for malignancy on cytology were found to contain frank malignant cells on review which may be ascribed to an error in initial screening of slides [Figure 1a–c].
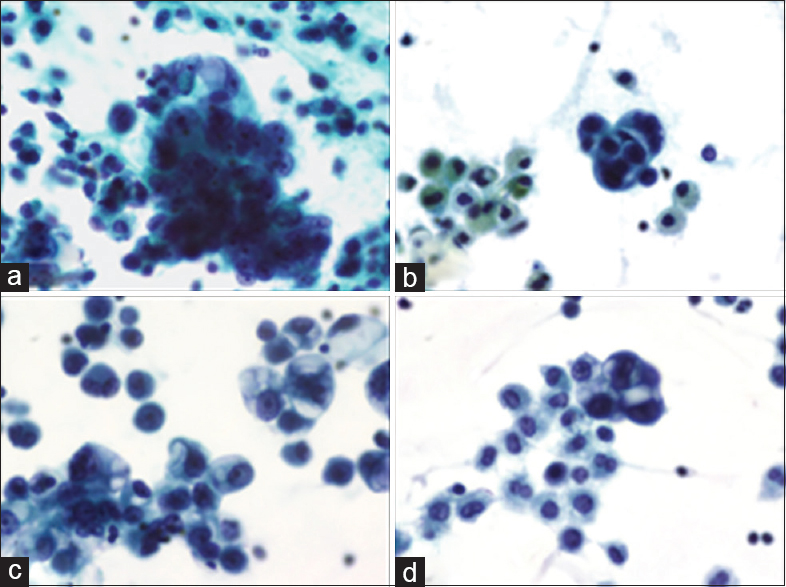
- (a) Hyperchromatic cells with prominent macronucleoli, a case of ovarian carcinoma initially labeled negative (PAP stain, ×400). (b) Cluster of malignant cells with bile pigment within macrophages on review of negative smear, a case of obstructive jaundice with massive ascites (PAP stain, ×400). (c) Vacuolated malignant cells with prominent nucleoli, a case of cecal adenocarcinoma (PAP stain, ×400). (d) Hyperchromatic cells with septate vacuoles in the cytoplasm with mesothelial cells on review of smear labeled atypical, a case of ovarian carcinoma (PAP stain, ×400)
A major diagnostic challenge lies in the distinction of atypical/reactive mesothelial cells from malignant adenocarcinoma cells. Smears from two patients in our study that were initially reported as atypical on cytology were probably misinterpreted due to overlapping cytomorphological features [Figure 1d]. The pattern of cells on low power and the cytomorphological details on high power may be a useful point for distinction. On low power, carcinoma cells may be present either singly or in small-to-large groups with complex three-dimensional papillary architecture. Their proliferation spheres show radial polarity at the periphery. Another important feature that helps in the recognition of malignancy is the presence of dual population of cells which can be appreciated at a low magnification.[18] On higher magnifications, these cells are characterized by sharply defined cytoplasmic vacuoles containing mucin which indents the nucleus giving rise to a signet ring morphology. These septate vacuoles show variation in size. This appearance may be mimicked by a poorly defined degenerative vacuole in a reactive mesothelial cell which can be distinguished on fine adjustment as the vacuole is found to overlap the nucleus and not indent it. Another point of distinction is that the nucleus in adenocarcinoma touches the cell periphery, whereas in a reactive mesothelial cell, there is a thin rim of cytoplasm that separates the nucleus from the cell membrane, and this feature has to be diligently searched for. The background cells in the smear also play an important role in deciding the benign nature of an effusion with reactive mesothelial cells. Cakir et al. reported that the presence of moderate-to-marked inflammatory background in 73.4% of their cases with reactive mesothelial proliferation in pleural effusion samples was benign in nature.[15] Ascitic fluid analysis is among the first few investigations asked for by the clinicians, but a detailed patient history and/or a radiology impression is often not available to the reporting cytologist. The term atypical cells is used in this context for want of detailed clinical information and can be misleading.
A comparison of data from similar publications over the past 10 years shows that the utility of conventional smear cytology is comparable [Table 2].[8913] A high false-negative rate up to 42% continues to be a challenge in using conventional smears alone for the diagnosis of malignancy by cytomorphology alone. Steps that can be taken to decrease the false negativity and improve the diagnostic accuracy are to include Diff-Quik (DQ) stain on air-dried smears and immunohistochemistry on formalin-fixed cell blocks. This Romanowsky stain, i.e., DQ stains the cytoplasm of the second population of foreign cells in the effusion differentially and highlights it more readily as compared to the Pap stain. It would be prudent to include this rapid, simple staining method in our laboratory on a routine basis for all suspected cases of malignant effusions. If the nuclear features of the second population of cells suggested by DQ method are not confirmed by the Pap stain, cell block is recommended. For objective interpretation, serial sections from cell block have to be identically oriented on the slides to enable precise mapping and evaluation of different cell types present by subtractive coordinate immunohistochemistry pattern (SCIP). In this method, the initial panel includes vimentin, pan-cytokeratin (a mixture of AE1/AE3 and CAM5.2), and PGM1 (CD68)/a mixture of LCA and PGM1 which highlight the mesothelial, epithelial, and inflammatory components of the smear, respectively. Along with calretinin and WT-1 (also mesothelial markers), it is possible to localize, identify, and characterize the second population of cells. Extreme care is required when interpreting which cells are positive. This approach of correlating and coordinating the immunoreactivity and nonimmunoreactivity is called SCIP evaluation.[19] Based on the clinical context, specific markers may be used for further characterization of the malignant cells. Cell block preparations would further be useful for tumor categorization which would be difficult by conventional smears alone.[2021] However, further evaluation is required as there are cost implications on performing these techniques in a low-resource setup.
| Study | Total cases (N) | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|
| Motherby et al., 1998[13] | 300 | 0.62 | 0.98 | 1 | 0.88 |
| Karoo et al., 2003[9] | 255 | 0.60 | 1 | 1 | 0.84 |
| Jha et al., 2006[8] | 65 | 0.56 | 1 | 1 | 0.63 |
| Present study, 2012 | |||||
| Before review | 115 | 0.59 | 1 | 1 | 0.77 |
| After review | 0.69 | 1 | 1 | 0.82 |
CONCLUSION
Despite recent advancements in diagnostic procedures, abdominal paracentesis for cytology will retain its place in the diagnostic armamentarium as a positive cytology report is usually conclusive of malignancy. Ascitic fluid cytology should be used judiciously for cases where there is a strong clinical suspicion for malignancy. A thorough morphological assessment with adequate clinical information and correlation with other investigations can be used to arrive at a definitive diagnosis in most cases. The term atypical cells can be misleading and is often used for want of clinical details and is best avoided.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. All authors have read and approved the final version of the manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study was approved by the Institutional Ethics Committee at Kasturba Medical College, Mangalore, where the study was conducted.
LIST OF ABBREVIATIONS (In alphabetic order)
DQ - Diff-Quik
FGT - Female genital tract
FNAC - Fine-needle aspiration cytology
HCC - Hepatocellular carcinoma
NPV - Negative predictive value
Pap - Papanicolaou
PPV - Positive predictive value
SAAG - Serum-ascites albumin gradient
SCIP - Subtractive coordinate immunohistochemistry pattern.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Ascitic fluid analysis with special reference to serum ascites cholesterol gradient and serum-ascites albumin gradient. Int J Res Sci. 2017;5:429-36.
- [Google Scholar]
- Ascitic fluid analysis in differential diagnosis of ascites: Focus on cirrhotic ascites. J Clin Transl Hepatol. 2014;2:58-64.
- [Google Scholar]
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417.
- [Google Scholar]
- The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215-20.
- [Google Scholar]
- Ascitic fluid analysis for the differentiation of malignancy-related and nonmalignant ascites. Proposal of a diagnostic sequence. Cancer. 1991;68:1808-14.
- [Google Scholar]
- Study of effusion cytology in patients with simultaneous malignancy and ascites. Kathmandu Univ Med J (KUMJ). 2006;4:483-7.
- [Google Scholar]
- How valuable is ascitic cytology in the detection and management of malignancy? Postgrad Med J. 2003;79:292-4.
- [Google Scholar]
- Malignant ascites: A review of prognostic factors, pathophysiology and therapeutic measures. World J Gastrointest Surg. 2012;4:87-95.
- [Google Scholar]
- Asystematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2012;15(Suppl 1):S27-37.
- [Google Scholar]
- Malignant ascites. Clinical and experimental observations. Ann Surg. 1986;203:644-51.
- [Google Scholar]
- Cytopathologic differential diagnosis of malignant mesothelioma, adenocarcinoma and reactive mesothelial cells: A logistic regression analysis. Diagn Cytopathol. 2009;37:4-10.
- [Google Scholar]
- Routine review of ascites fluid from patients with cirrhosis or hepatocellular carcinoma is a low-yield procedure: An observational study. Cytojournal. 2009;6:16.
- [Google Scholar]
- Challenges in the interpretation of peritoneal cytologic specimens. Arch Pathol Lab Med. 2009;133:739-42.
- [Google Scholar]
- Immunocytochemistry of effusion fluids: Introduction to SCIP approach. In: Shidham VB, Atkinson BF, eds. Cytopathologic Diagnosis of Serous Fluids (1st ed). Philadelphia, USA: Elsevier, W.B. Saunders Company; 2007. p. :55-78.
- [Google Scholar]
- Utility of cell block to detect malignancy in fluid cytology: Adjunct or necessity? J Cancer Res Ther. 2017;13:425-9.
- [Google Scholar]
- Cell blocks in cytopathology: A review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. 2014;25:356-71.
- [Google Scholar]








